|
DFT383
DFT383 is an investigational gene therapy under development by Novartis for the treatment of cystinosis, a rare autosomal recessive lysosomal storage disorder caused by mutations in the CTNS gene, leading to cystine accumulation in cells. The therapy delivers a functional copy of the CTNS gene via autologous hematopoietic stem cell (HSC) transplantation to address the disease’s root cause, targeting patients both pre- and post-kidney transplant. Mechanism of action DFT383 involves extracting a patient’s hematopoietic stem cells, genetically modifying them ex vivo with a lentiviral vector to insert a functional CTNS gene, and reinfusing them after myeloablation. The modified cells are intended to produce functional cystinosin, a lysosomal cystine-proton symporter, reducing cystine Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
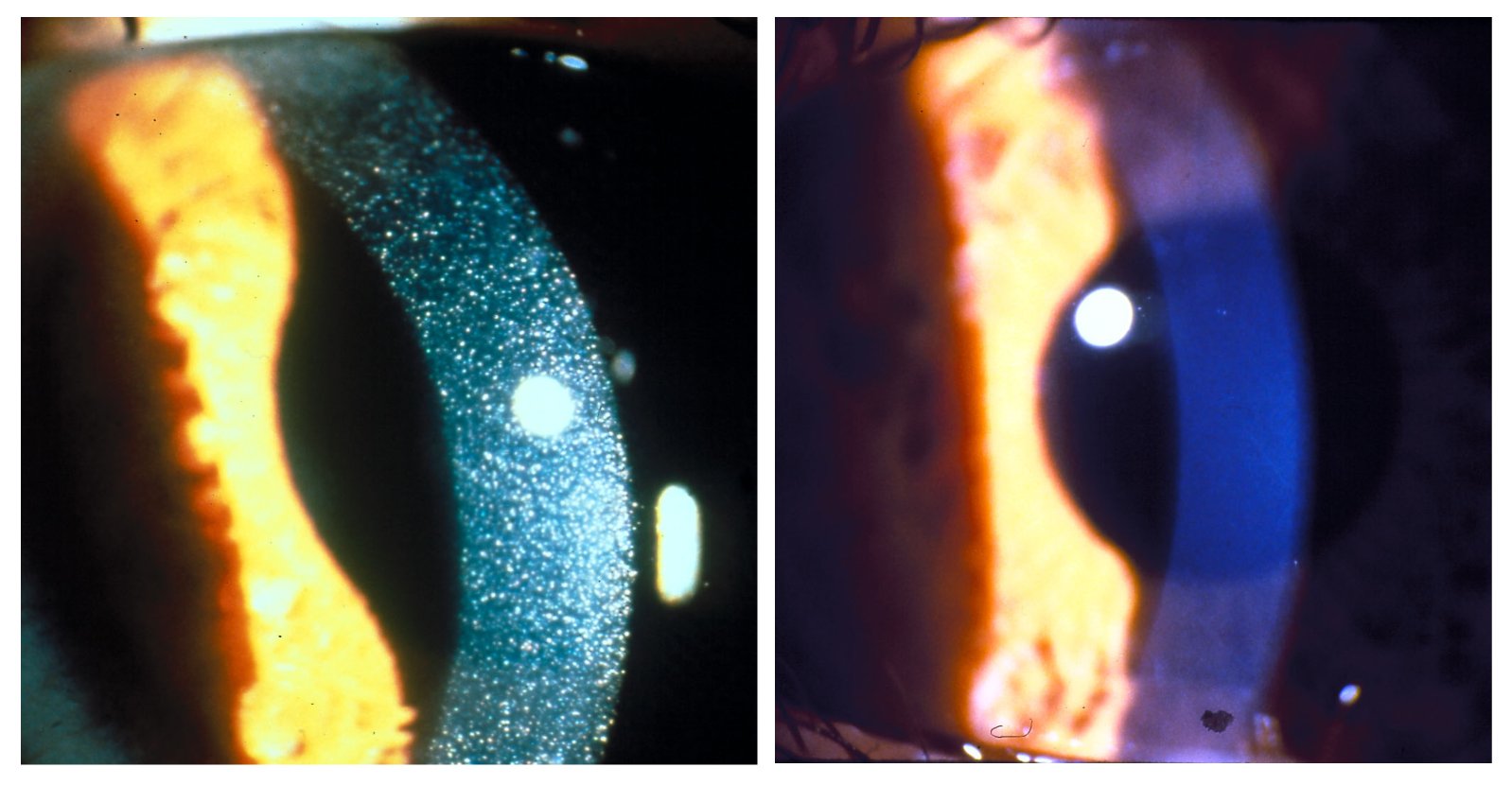
Cystinosis
Cystinosis is a lysosomal storage disease characterized by the abnormal accumulation of cystine, the oxidized dimer of the amino acid cysteine. It is a genetic disorder that follows an autosomal recessive inheritance pattern. It is a rare autosomal recessive disorder resulting from accumulation of free cystine in lysosomes, eventually leading to intracellular crystal formation throughout the body. Cystinosis is the most common cause of Fanconi syndrome in the pediatric age group. Fanconi syndrome occurs when the function of cells in renal tubules is impaired, leading to abnormal amounts of carbohydrates and amino acids in the urine, excessive urination, and low blood levels of potassium and phosphates. Cystinosis was the first documented genetic disease belonging to the group of lysosomal storage disease disorders.Nesterova G, Gahl WA. Cystinosis: the evolution of a treatable disease. Pediatr Nephrol 2012;28:51–9. Cystinosis is caused by mutations in the '' CTNS'' gene that code ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Therapy
Gene therapy is Health technology, medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells. The first attempt at modifying human DNA was performed in 1980, by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in Phases of clinical research, phase I. In 2003, Gendicine became the first gene therapy to receive regulatory approval. Since that time, further gene therapy drugs were approved, such as alipogene tiparvovec (2012), Strimvelis (2016), tisagenlecleucel (2017), voretigene neparvovec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novartis
Novartis AG is a Swiss multinational corporation, multinational pharmaceutical company, pharmaceutical corporation based in Basel, Switzerland. Novartis is one of the largest pharmaceutical companies in the world and was the eighth largest by revenue in 2024. Novartis manufactures the drugs clozapine (Clozaril), diclofenac (Voltaren; sold to GlaxoSmithKline in 2015 deal), carbamazepine (Tegretol), valsartan (Diovan), imatinib mesylate (Gleevec/Glivec), cyclosporine (Neoral/Sandimmune), letrozole (Femara), methylphenidate (Ritalin; produced by Sandoz since 2023), terbinafine (Lamisil), deferasirox (Exjade), and others. Novartis was formed in 1996 by the merger of Ciba-Geigy and Sandoz. It was considered the largest corporate merger in history during that time. The pharmaceutical and agrochemical divisions of both companies formed Novartis as an independent entity. The name Novartis was based on the Latin terms, ''novae artes'' (new skills). After the merger, other Ciba-Geigy and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystinosin
''CTNS may also refer to the Center for Theology and the Natural Sciences.'' ''CTNS'' is the gene that encodes the protein cystinosin in humans. Cystinosin is a lysosomal seven-transmembrane protein that functions as an active transporter for the export of cystine molecules out of the lysosome. Mutations in ''CTNS'' are responsible for cystinosis, an autosomal recessive lysosomal storage disease. Gene ''The CTNS'' gene is located on the p arm of human chromosome 17, at position 13.2. It spans base pairs 3,636,468 and 3,661,542, and comprises 12 exons. In 1995, the gene was localized to the short arm of chromosome 17. An international collaborative effort finally succeeded in isolating ''CTNS'' by positional cloning in 1998. The CTNSN323K, CTNSK280R, and CTNSN288K mutations completely stop the movement of CySS out of the lysosome via cystinosin. /sup> interestingly, CTNSN323K and CTNSK280R are related to juvenile nephropathic cystinosis while CTNSN288K mutations are found i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hematopoietic Stem Cell
Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm. Haematopoiesis is the process by which all mature blood cells are produced. It must balance enormous production needs (the average person produces more than 500 billion blood cells every day) with the need to regulate the number of each blood cell type in the circulation. In vertebrates, the vast majority of hematopoiesis occurs in the bone marrow and is derived from a limited number of hematopoietic stem cells that are multipotent and capable of extensive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lentiviral Vector
A viral vector is a modified virus designed to gene delivery, deliver genetic material into cell (biology), cells. This process can be performed inside an organism or in cell culture. Viral vectors have widespread applications in basic research, agriculture, and medicine. Viruses have evolved specialized molecular mechanisms to transport their genomes into infected hosts, a process termed transduction (genetics), transduction. This capability has been exploited for use as viral vectors, which may integrate their genetic cargo—the transgene—into the host genome, although non-integrative vectors are also commonly used. In addition to agriculture and laboratory research, viral vectors are widely applied in gene therapy: as of 2022, all approved gene therapies were viral vector-based. Further, compared to traditional vaccines, the intracellular antigen expression enabled by viral vector vaccines offers more robust immune activation. Many types of viruses have been developed into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symporter
A symporter is an integral membrane protein that is involved in the transport of two (or more) different molecules across the cell membrane in the same direction. The symporter works in the plasma membrane and molecules are transported across the cell membrane at the same time, and is, therefore, a type of cotransporter. The transporter is called a symporter, because the molecules will travel in the same direction in relation to each other. This is in contrast to the antiport transporter. Typically, the ion(s) will move down the electrochemical gradient, allowing the other molecule(s) to move against the concentration gradient. The movement of the ion(s) across the membrane is facilitated diffusion, and is coupled with the active transport of the molecule(s). In symport, two molecule move in a 'similar direction' at the 'same time'. For example, the movement of glucose along with sodium ions. It exploits the uphill movement of other molecules from low to high concentration, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist in rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and whole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase I Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Description Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. When expressed specifically, a clinical trial phase is capitalized both in name and Roman numeral, such as "Phase I" clinical trial. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory auth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Description Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. When expressed specifically, a clinical trial phase is capitalized both in name and Roman numeral, such as "Phase I" clinical trial. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory aut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteamine
Cysteamine is an organosulfur compound with the formula . A white, water-soluble solid, it contains both an amine and a thiol functional group. It is often used as the salt of the ammonium derivative SCH2CH2NH3sup>+, including the hydrochloride, phosphocysteamine, and the bitartrate. The intermediate pantetheine is broken down into cysteamine and pantothenic acid. It is biosynthesized in mammals, including humans, by the degradation of coenzyme A. It is the biosynthetic precursor to the neurotransmitter hypotaurine. Medical uses As a medication sold under the brand names ''Procysbi'' or ''Cystagon'', among others, cysteamine is indicated to treat cystinosis, a lysosomal storage disease characterized by the abnormal accumulation of cystine, the oxidized dimer of the amino acid cysteine. It removes the excessive cystine that builds up in cells of people with the disease. It is available by mouth (capsule and extended release capsule) and in eye drops. When applied topic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |