|
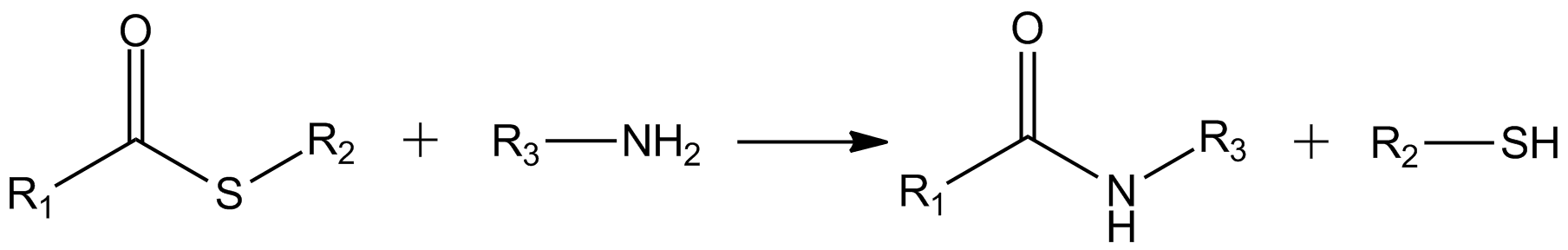
Thioesters
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : The analogous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compounds
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, Desulfurization, the removal of which is a Claus process, major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a Substrate (chemistry), substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenic acid, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In acetyl-CoA, its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the Anabolism, anabolic and Catabolism, catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of Pyruvic acid, pyruvate synthesis and degradation. Discovery of structure Coenzyme A was ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a cysteamine, β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through Beta oxidation, β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desiccant
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams. Types of desiccants Although some desiccants are chemically inert, others are extremely reactive and require specialized handling techniques. The most common desiccant is silica gel, an otherwise inert, nontoxic, water-insoluble white solid. Tens of thousands of tons are produced annually for this purpose. Other common desiccants include activated charcoal, calcium sulfate, calcium chloride, and molecular sieves (typically, zeolites). Desicc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N'-Dicyclohexylcarbodiimide
is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water. Structure and spectroscopy The C−N=C=N−C core of carbodiimides (N=C=N) is linear, being related to the structure of allene. The molecule has idealized C2 symmetry. The N=C=N moiety gives characteristic IR spectroscopic signature at 2117 cm−1. The 15N NMR spectrum shows a characteristic shift of 275 ppm upfield of nitric acid and the 13C NMR spectrum features a peak at about 139 ppm downfield from TMS. Preparation DCC is produced by the decarboxylation of cyclohexylisocyanate using phosphine oxides as a catalyst: :2 C6H11NCO → (C6H11N)2C + CO2 Alternative catalysts for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanephosphonic Acid Anhydride
Propanephosphonic acid anhydride (PPAA, T3P) is an anhydride of propane phosphonic acid. Its structure is a cyclic trimer, with a phosphorus–oxygen core and propyl groups and additional oxygens attached. The chemical is a useful reagent for peptide synthesis reactions, where it activates the carboxylic acid partner for subsequent reaction with amines. It is commercially available as 50 % solution in DMF or ethyl acetate Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ... as a slightly yellow mixture. References Reagents for organic chemistry Phosphonates {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
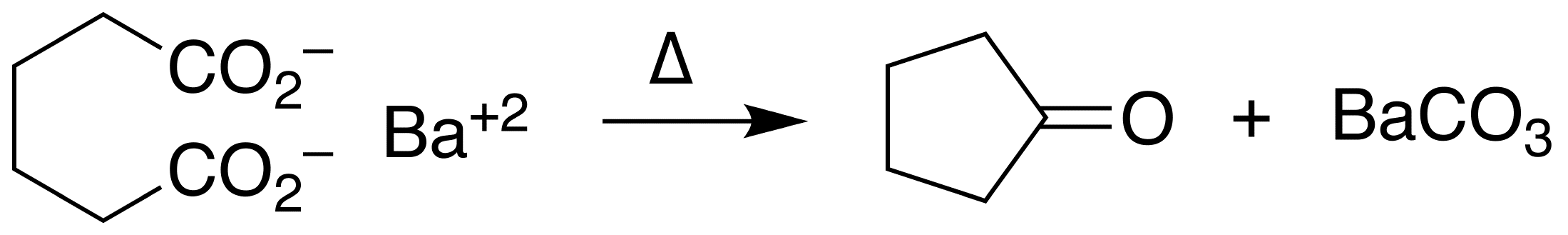
Cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid. Preparation Ketonic decarboxylation of adipic acid gives cyclopentanone. The reaction is conducted at elevated temperatures in the presence of barium hydroxide. : The Pd-catalyzed oxidation of cyclopentene also gives cyclopentanone. Uses Cyclopentanone is common precursor to fragrances, especially those related to jasmine and jasmone. Examples include 2-pentyl- and 2-heptylcyclopentanone. It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital. Cyclopentanone is also used to make cyclopentamine, the pesticide pencycuron, and pentethylcyclanone. It is also used as a precursor to cubane-1,4-dicarboxylate, which is used to synthesize other substituted cubanes, such as the high explosives heptanitrocubane and octanitrocubane. References {{Authority control Cycloalkanones, 5 Ketone solvents Perfume ingredients Cyclopentanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid. In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction. In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. See also * Base anhydride, an oxide that reacts with water to form a hydroxide Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ... salt References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Thioacetate
Potassium thioacetate is an organosulfur compound and a salt with the formula . This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives. Synthesis and reactions Potassium thioacetate, which is commercially available, can be prepared by combining acetyl chloride and potassium hydrogen sulfide: : It arises also by the neutralization of thioacetic acid with potassium hydroxide. Use in preparation of thiols In a common application, potassium thioacetate is combined with alkylating agents to give thioacetate esters (X = halide): : Hydrolysis of these esters affords thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...s: : The thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as ill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitsunobu Reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). In a typical protocol, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent (e.g. diethyl ether), cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |