thioesters on:
[Wikipedia]
[Google]
[Amazon]
In
RSNa + R'COCl -> R'COSR + NaCl
Another common route entails the displacement of CH3COSK + RX -> CH3COSR + KX
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using Mannich bases and the thiocarboxylic acid:
:CH3COSH + R'2NCH2OH -> CH3COSCH2NR'2 + H2O
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of dehydrating agents:
:RSH + R'CO2H -> RSC(O)R' + H2O
A typical dehydration agent is DCC. Efforts to improve the sustainability of thioester synthesis have also been reported utilising safer coupling reagent T3P and greener solvent
 In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an
In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, thioesters are organosulfur compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur ...
with the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
. They are analogous to carboxylate ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the '' thio-'' prefix. They are the product of esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glyceride ...
between a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
() and a thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
(). In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology ...
, the best-known thioesters are derivatives of coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a subs ...
, e.g., acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized fo ...
.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764.
Synthesis
The most typical route to thioester involves the reaction of anacid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
with an alkali metal salt of a thiol:
:halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
s by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
of potassium thioacetate:
:cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclope ...
. Acid anhydride An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equiva ...
s and some lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s also give thioesters upon treatment with thiols in the presence of a base.
Thioesters can be conveniently prepared from alcohols by the Mitsunobu reaction, using thioacetic acid.
They also arise via carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
of alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
s and alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s in the presence of thiols.
Reactions
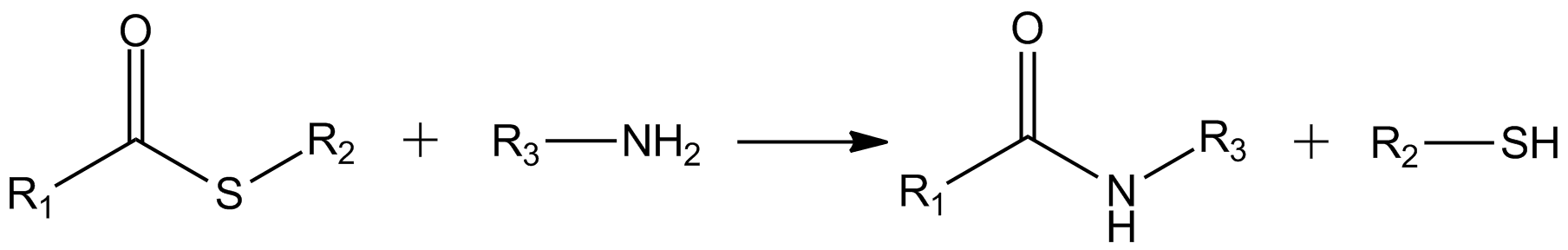
Thioesters hydrolyze to thiols and the carboxylic acid: :RC(O)SR' + H2O → RCO2H + RSH The carbonyl center in thioesters is more reactive toward amine nucleophiles to giveamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s:
: In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an
In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compoun ...
by a palladium catalyst to give a ketone.
:
Biochemistry
Thioesters are common intermediates in many biosynthetic reactions, including the formation and degradation offatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s and mevalonate, precursor to steroids. Examples include malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and com ...
, acetoacetyl-CoA
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis ( ketogenesis) pathway of the liver. In the ketone bodies digesti ...
, propionyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several diffe ...
, cinnamoyl-CoA
Cinnamoyl-Coenzyme A is an intermediate in the phenylpropanoids metabolic pathway.
Enzymes using Cinnamoyl-Coenzyme A
* Cinnamoyl-CoA reductase, an enzyme that catalyzes the chemical reaction cinnamaldehyde + CoA + NADP+ → cinnamoyl-CoA + NA ...
, and acyl carrier protein (ACP) thioesters. Acetogenesis Acetogenesis is a process through which acetate is produced either by the reduction of CO2 or by the reduction of organic acids, rather than by the oxidative breakdown of carbohydrates or ethanol, as with acetic acid bacteria.
The different bact ...
proceeds via the formation of acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized fo ...
. The biosynthesis of lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity ...
, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of caffeic acid
Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, o ...
. These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. F ...
, which tags the protein for degradation.
Oxidation of the sulfur atom in thioesters (thiolactones) is postulated in the bioactivation of the antithrombotic prodrugs ticlopidine, clopidogrel, and prasugrel.
Thioesters and the origin of life
As posited in a "Thioester World", thioesters are possible precursors to life. AsChristian de Duve
Christian René Marie Joseph, Viscount de Duve (2 October 1917 – 4 May 2013) was a Nobel Prize-winning Belgian cytologist and biochemist. He made serendipitous discoveries of two cell organelles, peroxisome and lysosome, for which he shared ...
explains:
It is revealing that thioesters are obligatory intermediates in several key processes in whichATP ATP may refer to: Companies and organizations * Association of Tennis Professionals, men's professional tennis governing body * American Technical Publishers, employee-owned publishing company * ', a Danish pension * Armenia Tree Project, non ...is either used or regenerated. Thioesters are involved in the synthesis of allesters In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ..., including those found in complexlipid Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids incl ...s. They also participate in the synthesis of a number of other cellular components, includingpeptide Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. ...