Cyclopentanone on:
[Wikipedia]
[Google]
[Amazon]
Cyclopentanone is the
 The Pd-catalyzed oxidation of
The Pd-catalyzed oxidation of
 Cyclopentanone is also used to make
Cyclopentanone is also used to make
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with the formula (CH2)4CO. This cyclic ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
is a colorless volatile liquid.
Preparation
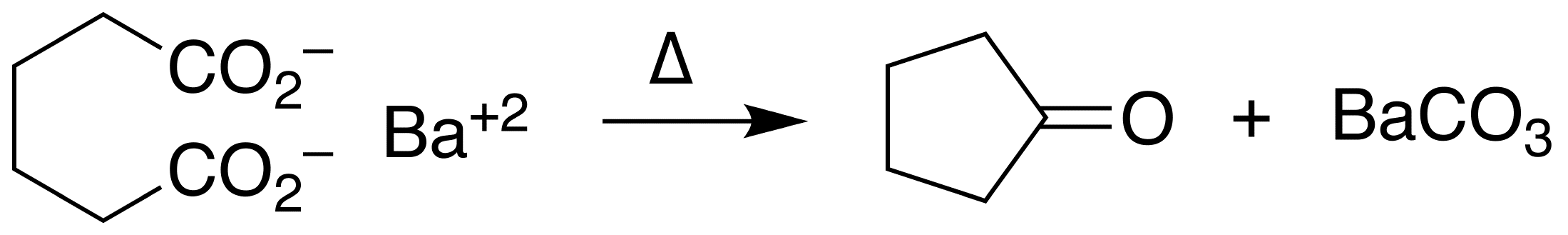
Ketonic decarboxylation
Ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction involving decarboxylation, converting two equivalents of a carboxylic acid () to a symmetric ketone (). The reaction typically requires heat and a ...
of adipic acid
Adipic acid or hexanedioic acid is the organic compound with the formula C6H10O4. It a white crystalline powder at standard temperature and pressure. From an industrial perspective, it is the most important dicarboxylic acid at about 2.5 billion ...
gives cyclopentanone. The reaction is conducted at elevated temperatures in the presence of barium hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
...
.
: The Pd-catalyzed oxidation of
The Pd-catalyzed oxidation of cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of t ...
also gives cyclopentanone.
Uses
Cyclopentanone is common precursor to fragrances, especially those related tojasmine
Jasmine (botanical name: ''Jasminum'', pronounced ) is a genus of shrubs and vines in the olive family of Oleaceae. It contains around 200 species native to tropical and warm temperate regions of Eurasia, Africa, and Oceania. Jasmines are wid ...
and jasmone
Jasmone is an organic compound, which is a volatile portion of the oil from jasmine flowers. It is a colorless to pale yellow liquid. Jasmone can exist in two isomeric forms with differing geometry around the pentenyl double bond, ''cis''-jasmon ...
. Examples include 2-pentyl- and 2-heptylcyclopentanone. It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital
Cyclopentobarbital sodium (Cyclopal, Dormisan) is a barbiturate derivative invented in the 1940s. It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine. Cyclopal is considered similar in ...
.
cyclopentamine
Cyclopentamine (trade names Clopane, Cyclonarol, Cyclosal, Cyklosan, Nazett, Sinos, among others) is a sympathomimetic and vasoconstrictor drug of the alkylamine family and related to the arylalkylamines. Cyclopentamine was indicated in the pa ...
, the pesticide pencycuron, and pentethylcyclanone
Pentethylcyclanone is an antitussive medication
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to medical diagnosis, diagnose, cure, treat, or preventi ...
.
It is also used as a precursor to cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substanc ...
-1,4-dicarboxylate, which is used to synthesize other substituted cubanes, such as the high explosives heptanitrocubane
Heptanitrocubane is an experimental high explosive based on the cubic eight-carbon cubane molecule and closely related to octanitrocubane. Seven of the eight hydrogen atoms at the corners of the cubane molecule are replaced by nitro groups, giv ...
and octanitrocubane
Octanitrocubane (molecular formula: C8(NO2)8) is a proposed high explosive that, like TNT, is shock-insensitive (not readily detonated by shock). The octanitrocubane molecule has the same chemical structure as cubane (C8H8) except that each of th ...
.
References
{{Authority control 5 Ketone solvents Perfume ingredients Cyclopentanes