|
Critical Micelle Concentration
In colloidal chemistry, colloidal and surface chemistry, the critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system will form micelles. The CMC is an important characteristic of a surfactant. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant or changes with a lower slope. The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence and concentration of other surface active substances and electrolytes. Micelles only form above Krafft temperature, critical micelle temperature. For example, the value of CMC for sodium dodecyl sulfate in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10−3 mol/L. Description Upon introducing surfactants (or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloidal Chemistry
Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with ''colloids'', heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a Solution (chemistry), true solution and a suspension (chemistry), suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane. Interface and colloid science has applications and ramifications in the chemical industry, pharmaceuticals, biotechnology, ceramics, minerals, nanotec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
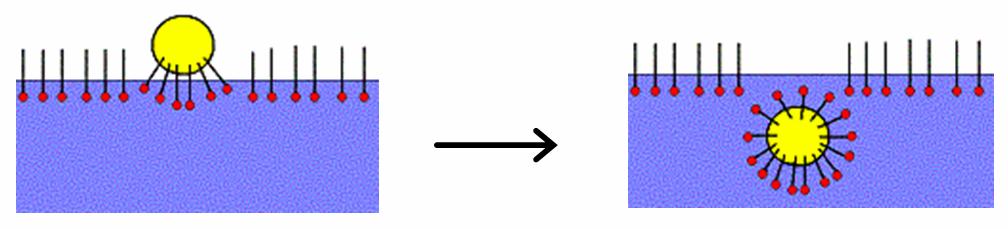
Micelle
A micelle () or micella () ( or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). A typical micelle in water forms an aggregate, with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. The difficulty in filling the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (or oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (or water-in-oil micelle). Micelles are approximately spherical in shape. Other shapes, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonates, which are soap-like compounds that are more soluble than soap in hard water, because the polar sulfonate is less likely than the polar carboxylate of soap to bind to calcium and other ions found in hard water. Definitions The word ''detergent'' is derived from the Latin adjective , from the verb , meaning to wipe or polish off. Detergent can be defined as a surfactant or a mixture of surfactants with cleansing properties when in Concentration, dilute Solution (chemistry), solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to ''soap'' (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term ''detergent'' refers to househ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enhanced Oil Recovery
Enhanced oil recovery (abbreviated EOR), also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted after primary and secondary recovery methods have been completely exhausted. Whereas primary and secondary recovery techniques rely on the pressure differential between the surface and the underground well, enhanced oil recovery functions by altering the physical or chemical properties of the oil itself in order to make it easier to extract. When EOR is used, 30% to 60% or more of a reservoir's oil can be extracted, compared to 20% to 40% using only Extraction of petroleum#Primary recovery, primary and Extraction of petroleum#Secondary recovery, secondary recovery. There are four main EOR techniques: carbon dioxide (CO2) injection, gas injection, thermal EOR, and chemical EOR. More advanced, speculative EOR techniques are sometimes called quaternary recovery. Carbon dioxide injection, known as CO2-EOR, is the most common method. In thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubilization
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solubilization) into or onto micelles. Solubilization may occur in a system consisting of a solvent, an association colloid (a colloid that forms micelles), and at least one other solubilizate. Usage of the term Solubilization is distinct from dissolution because the resulting fluid is a colloidal dispersion involving an association colloid. This suspension is distinct from a true solution, and the amount of the solubilizate in the micellar system can be different (often higher) than the regular solubility of the solubilizate in the solvent. In non-chemical literature and in everyday language, the term "solubilization" is sometimes used in a broader meaning as "to bring to a solution or (non- sedimenting) suspension" by any means, e.g., leaching by a reaction with an acid. Application Micellar solubilization is widely utilized, e.g. in laundry washing usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonates, which are soap-like compounds that are more soluble than soap in hard water, because the polar sulfonate is less likely than the polar carboxylate of soap to bind to calcium and other ions found in hard water. Definitions The word ''detergent'' is derived from the Latin adjective , from the verb , meaning to wipe or polish off. Detergent can be defined as a surfactant or a mixture of surfactants with cleansing properties when in Concentration, dilute Solution (chemistry), solutions. However, conventionally, detergent is used to mean synthetic cleaning compounds as opposed to ''soap'' (a salt of the natural fatty acid), even though soap is also a detergent in the true sense. In domestic contexts, the term ''detergent'' refers to househ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Micelle Concentration (CMC) Curve Attension Sigma 701
In colloidal and surface chemistry, the critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system will form micelles. The CMC is an important characteristic of a surfactant. Before reaching the CMC, the surface tension changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant or changes with a lower slope. The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence and concentration of other surface active substances and electrolytes. Micelles only form above critical micelle temperature. For example, the value of CMC for sodium dodecyl sulfate in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10−3 mol/L. Description Upon introducing surfactants (or any surface active materials) into a sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase (matter), phase) is dispersion (chemistry), dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tensiometer (surface Tension)
In surface science, a tensiometer is a measuring instrument used to measure the surface tension () of liquids or surfaces. Tensiometers are used in research and development laboratories to determine the surface tension of liquids like coatings, lacquers or adhesives. A further application field of tensiometers is the monitoring of industrial production processes like parts cleaning or electroplating. Types Goniometer/Tensiometer Surface scientists commonly use an optical goniometer/tensiometer to measure the surface tension and interfacial tension of a liquid using the pendant or sessile drop methods. A drop is produced and captured using a CCD camera. The drop profile is subsequently extracted, and sophisticated software routines then fit the theoretical Young-Laplace equation to the experimental drop profile. The surface tension can then be calculated from the fitted parameters. Unlike other methods, this technique requires only a small amount of liquid making it suitable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflection Point
In differential calculus and differential geometry, an inflection point, point of inflection, flex, or inflection (rarely inflexion) is a point on a smooth plane curve at which the curvature changes sign. In particular, in the case of the graph of a function, it is a point where the function changes from being concave (concave downward) to convex (concave upward), or vice versa. For the graph of a function of differentiability class (its first derivative , and its second derivative , exist and are continuous), the condition can also be used to find an inflection point since a point of must be passed to change from a positive value (concave upward) to a negative value (concave downward) or vice versa as is continuous; an inflection point of the curve is where and changes its sign at the point (from positive to negative or from negative to positive). A point where the second derivative vanishes but does not change its sign is sometimes called a point of undulation or und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |