|
Chromium Difluoride
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air. Preparation and structure The compound is prepared by passing anhydrous hydrogen fluoride over anhydrous chromium(II) chloride. The reaction will proceed at room temperature but is typically heated to 100-200 °C to ensure completion: :CrCl2 + 2 HF → CrF2 + 2 HCl Like many difluorides, CrF2 adopts a structure like rutile with octahedral molecular geometry about Cr(II) and trigonal geometry at F−. Two of the six Cr–F bonds are long at 2.43 Å, and four are short near 2.00 Å. This distortion is a consequence of the Jahn–Teller effect that arises from the d4 elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal. Chromium is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polishing, polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared, infrared light. The name of the element is derived from the Ancient Greek, Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Chloride
Chromium(II) chloride describes inorganic compounds with the formula Cr Cl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes. Synthesis CrCl2 is produced by reducing chromium(III) chloride either with hydrogen at 500 °C: :2CrCl3 + H2 → 2CrCl2 + 2HCl or by electrolysis. On the laboratory scale, LiAlH4, zinc, and related reductants produce chromous chloride from chromium(III) precursors: :4 CrCl3 + LiAlH4 → 4 CrCl2 + LiCl + AlCl3 + 2 H2 :2 CrCl3 + Zn → 2 CrCl2 + ZnCl2 CrCl2 can also be prepared by treating a solution of chromium(II) acetate with hydrogen chloride: :Cr2(OAc)4 + 4 HCl → 2 CrCl2 + 4 AcOH Treatment of chromium powder with concentrated hydrochloric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Compounds
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal. Chromium is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polishing, polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared, infrared light. The name of the element is derived from the Ancient Greek, Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Chloride
Chromium(II) chloride describes inorganic compounds with the formula Cr Cl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes. Synthesis CrCl2 is produced by reducing chromium(III) chloride either with hydrogen at 500 °C: :2CrCl3 + H2 → 2CrCl2 + 2HCl or by electrolysis. On the laboratory scale, LiAlH4, zinc, and related reductants produce chromous chloride from chromium(III) precursors: :4 CrCl3 + LiAlH4 → 4 CrCl2 + LiCl + AlCl3 + 2 H2 :2 CrCl3 + Zn → 2 CrCl2 + ZnCl2 CrCl2 can also be prepared by treating a solution of chromium(II) acetate with hydrogen chloride: :Cr2(OAc)4 + 4 HCl → 2 CrCl2 + 4 AcOH Treatment of chromium powder with concentrated hydrochloric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromyl Fluoride
Chromyl fluoride is an inorganic compound with the formula . It is a violet-red colored crystalline solid that melts to an orange-red liquid.Gard, G. L. (1986) "Chromium Difluoride Dioxide (Chromyl Fluoride)," '' Inorg. Synth.'', 24, 67-69, . Structure The liquid and gaseous have a tetrahedral geometry with C2v symmetry, much like chromyl chloride. Chromyl fluoride dimerizes via fluoride bridges (as ) in the solid state, crystallizing in the P21/c space group with Z = 4. The Cr=O bond lengths are about 157 pm, and the Cr–F bond lengths are 181.7, 186.7, and 209.4 pm. Chromium resides in a distorted octahedral position with a coordination number of 6. History and preparation Pure chromyl fluoride was first isolated in 1952 as reported by Alfred Engelbrecht and Aristid von Grosse.Engelbrecht, A.; von Grosse, A. (1952) "Pure Chromyl Fluoride," '' J. Am. Chem. Soc.'' 74(''21''), 5262–5264, . It was first observed as red vapor in the early 19th century upon heatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D Electron Count
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory. However the d electron count of an atom in a complex is often different from the d electron count of a free atom or a free ion of the same element. Electron configurations of transition metal atoms For free atoms, electron configurations have been determined by atomic spectroscopy. Lists of atomic energy levels and their electron configurations have been published by the National Institute of Standards and Technology (NIST) for both neutral and ionized atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jahn–Teller Effect
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in spectroscopy, stereochemistry, crystal chemistry, molecular and solid-state physics, and materials science. The effect is named for Hermann Arthur Jahn and Edward Teller, who first reported studies about it in 1937. Simplified overview The Jahn–Teller effect, sometimes also referred to as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that results from certain electron configurations. The Jahn–Teller theorem essentially states that any non-linear molecule with a spatially degenerate energy level, degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite. Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum. Rutile derives its name from the Latin ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. Occurrence Rutile is a comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
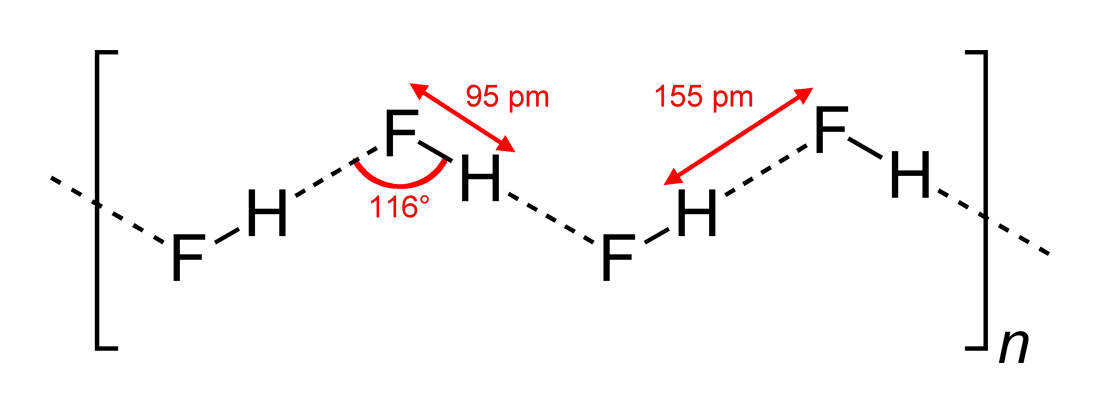
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Oxide
Chromium(III) oxide (or chromia) is an inorganic compound with the formula . It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite. Structure and properties has the corundum structure, consisting of a hexagonal close packed array of oxide anions with two thirds of the octahedral holes occupied by chromium. Similar to corundum, is a hard, brittle material (Mohs hardness 8 to 8.5). It is antiferromagnetic up to , the Néel temperature. It is not readily attacked by acids. Occurrence occurs naturally as the mineral eskolaite, which is found in chromium-rich tremolite skarns, metaquartzites, and chlorite veins. Eskolaite is also a rare component of chondrite meteorites. The mineral is named after Finnish geologist Pentti Eskola. Production The Parisians Pannetier and Binet first prepared the transparent hydrated form of in 1838 via a secret process, sold as a pigment. It is derived from the mineral chromi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |