Rutile on:
[Wikipedia]
[Google]
[Amazon]
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10%
 Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment, particularly as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in
Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment, particularly as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in  The occurrence of large specimen crystals is most common in pegmatites, skarns, and
The occurrence of large specimen crystals is most common in pegmatites, skarns, and
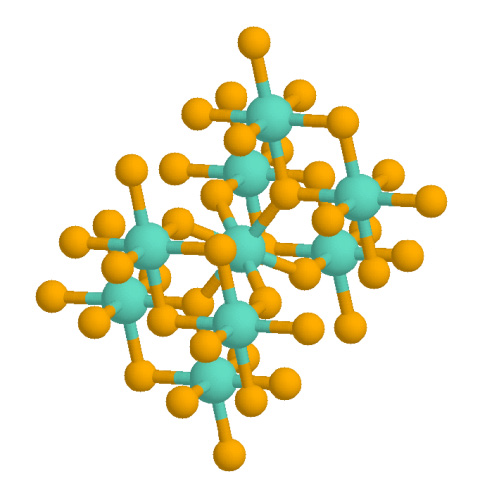 The structure of rutile is so classic that it is discussed in textbooks as a reference motif, much like
The structure of rutile is so classic that it is discussed in textbooks as a reference motif, much like
 In large enough quantities in beach sands, rutile forms an important constituent of heavy minerals and ore deposits. Miners extract and separate the valuable minerals – e.g., rutile, zircon, and ilmenite. The main uses for rutile are the manufacture of refractory ceramic, as a
In large enough quantities in beach sands, rutile forms an important constituent of heavy minerals and ore deposits. Miners extract and separate the valuable minerals – e.g., rutile, zircon, and ilmenite. The main uses for rutile are the manufacture of refractory ceramic, as a
Magnetism in titanium dioxide polymorphs
J. Applied Physics Research efforts typically utilize small quantities of synthetic rutile rather than mineral-deposit derived materials.
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
and significant amounts of niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
and tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
.
Rutile derives its name from the Latin ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality.
Occurrence
Rutile is a common accessory mineral in high-temperature and high-pressure metamorphic rocks and in igneous rocks. Thermodynamically, rutile is the most stable polymorph of TiO2 at all temperatures, exhibiting lower total free energy than metastable phases of anatase or brookite. Consequently, the transformation of the metastable TiO2 polymorphs to rutile is irreversible. As it has the lowest molecular volume of the three main polymorphs, it is generally the primary titanium-bearing phase in most high-pressure metamorphic rocks, chiefly eclogites. Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment, particularly as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in
Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment, particularly as products of autogenic alteration during the cooling of plutonic rocks; anatase is also found in placer deposit
In geology, a placer deposit or placer is an accumulation of valuable minerals formed by gravity separation from a specific source rock during sedimentary processes. The name is from the Spanish language, Spanish word ''placer'', meaning "alluviu ...
s sourced from primary rutile.
 The occurrence of large specimen crystals is most common in pegmatites, skarns, and
The occurrence of large specimen crystals is most common in pegmatites, skarns, and granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly coo ...
greisens. Rutile is found as an accessory mineral in some altered igneous rocks, and in certain gneisses and schist
Schist ( ) is a medium-grained metamorphic rock generally derived from fine-grained sedimentary rock, like shale. It shows pronounced ''schistosity'' (named for the rock). This means that the rock is composed of mineral grains easily seen with a l ...
s. In groups of acicular crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s it is frequently seen penetrating quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
as in the from Graubünden, Switzerland
Switzerland, officially the Swiss Confederation, is a landlocked country located in west-central Europe. It is bordered by Italy to the south, France to the west, Germany to the north, and Austria and Liechtenstein to the east. Switzerland ...
. In 2005 the Republic of Sierra Leone
Sierra Leone, officially the Republic of Sierra Leone, is a country on the southwest coast of West Africa. It is bordered to the southeast by Liberia and by Guinea to the north. Sierra Leone's land area is . It has a tropical climate and envi ...
in West Africa
West Africa, also known as Western Africa, is the westernmost region of Africa. The United Nations geoscheme for Africa#Western Africa, United Nations defines Western Africa as the 16 countries of Benin, Burkina Faso, Cape Verde, The Gambia, Gha ...
had a production capacity of 23% of the world's annual rutile supply, which rose to approximately 30% in 2008.
Crystal structure
 The structure of rutile is so classic that it is discussed in textbooks as a reference motif, much like
The structure of rutile is so classic that it is discussed in textbooks as a reference motif, much like sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
and nickel arsenide. The structure is adopted by not only TiO2, but also by GeO2, RuO2, SnO2, MnO2, VO2, IrO2, and CrO2. Somewhat curiously, ZrO2 and HfO2 adopt another classical structural motif, the fluorite structure.
In the rutile motif, the metal "cations" have a coordination number of 6, meaning they are surrounded by an octahedron of 6 oxygen atoms. The oxygen anions have a coordination number of 3, in a trigonal planar coordination. Rutile also shows a screw axis when its octahedra are viewed sequentially. When formed under reducing conditions, oxygen vacancies can occur, coupled to Ti3+ centers. Hydrogen can enter these gaps, existing as an individual vacancy occupant (pairing as a hydrogen ion) or creating a hydroxide group with an adjacent oxygen.
Rutile crystals are most commonly observed to exhibit a prismatic or acicular growth habit with preferential orientation along their ''c'' axis, the 01 direction. This growth habit is favored as the facets of rutile exhibit the lowest surface free energy and are therefore thermodynamically most stable. The ''c''-axis oriented growth of rutile appears clearly in nanorods, nanowires and abnormal grain growth phenomena of this phase.
Application
 In large enough quantities in beach sands, rutile forms an important constituent of heavy minerals and ore deposits. Miners extract and separate the valuable minerals – e.g., rutile, zircon, and ilmenite. The main uses for rutile are the manufacture of refractory ceramic, as a
In large enough quantities in beach sands, rutile forms an important constituent of heavy minerals and ore deposits. Miners extract and separate the valuable minerals – e.g., rutile, zircon, and ilmenite. The main uses for rutile are the manufacture of refractory ceramic, as a pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
, and for the production of titanium metal.
Finely powdered rutile is a brilliant white pigment and is used in paints, plastic
Plastics are a wide range of synthetic polymers, synthetic or Semisynthesis, semisynthetic materials composed primarily of Polymer, polymers. Their defining characteristic, Plasticity (physics), plasticity, allows them to be Injection moulding ...
s, paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
, foods, and other applications that call for a bright white color. Titanium dioxide pigment is the single greatest use of titanium worldwide. Nanoscale particles of rutile are transparent to visible light but are highly effective in the absorption of ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
radiation ( sunscreen). The UV absorption of nano-sized rutile particles is blue-shifted compared to bulk rutile so that higher-energy UV light is absorbed by the nanoparticles. Hence, they are used in sunscreens to protect against UV-induced skin damage.
Small rutile needles present in gems are responsible for an optical phenomenon known as asterism. Asteriated gems are known as "star" gems. Star sapphire
Sapphire is a precious gemstone, a variety of the mineral corundum, consisting of aluminium oxide () with trace amounts of elements such as iron, titanium, cobalt, lead, chromium, vanadium, magnesium, boron, and silicon. The name ''sapphire ...
s, star rubies, and other star gems are highly sought after and are generally more valuable than their normal counterparts.
Rutile is widely used as a welding electrode covering. It is also used as a part of the ZTR index, which classifies highly weathered sediments.
Semiconductor
Rutile, as a large band-gapsemiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
, has in recent decades been the subject of significant research towards applications as a functional oxide for applications in photocatalysis and dilute magnetism.J. Applied Physics Research efforts typically utilize small quantities of synthetic rutile rather than mineral-deposit derived materials.
Synthetic rutile
Synthetic rutile was first produced in 1948 and is sold under a variety of names. It can be produced from the titanium ore ilmenite through the Becher process. Very pure synthetic rutile is transparent and almost colorless, being slightly yellow, in large pieces. Synthetic rutile can be made in a variety of colors by doping. The highrefractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
gives an adamantine luster and strong refraction that leads to a diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
-like appearance. The near-colorless diamond substitute is sold as "Titania", which is the old-fashioned chemical name for this oxide. However, rutile is seldom used in jewellery
Jewellery (or jewelry in American English) consists of decorative items worn for personal adornment such as brooches, ring (jewellery), rings, necklaces, earrings, pendants, bracelets, and cufflinks. Jewellery may be attached to the body or the ...
because it is not very hard (scratch-resistant), measuring only about 6 on the Mohs hardness scale.
As the result of growing research interest in the photocatalytic activity of titanium dioxide, in both anatase and rutile phases (as well as biphasic mixtures of the two phases), rutile TiO2 in powder and thin film form is frequently fabricated in laboratory conditions through solution based routes using inorganic precursors (typically TiCl4) or organometallic precursors (typically alkoxides such as titanium isopropoxide, also known as TTIP). Depending on synthesis conditions, the first phase to crystallize may be the metastable anatase phase, which can then be converted to the equilibrium rutile phase through thermal treatment. The physical properties of rutile are often modified using dopants
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optical properties. The amount of dopant is typically very low compared to the material being do ...
to impart improved photocatalytic activity through improved photo-generated charge carrier separation, altered electronic band structures and improved surface reactivity.
See also
* List of mineralsReferences
External links
* {{Authority control Titanium minerals Oxide minerals Rutile group Tetragonal minerals Minerals in space group 136 Minerals described in 1803