|
Chromatography
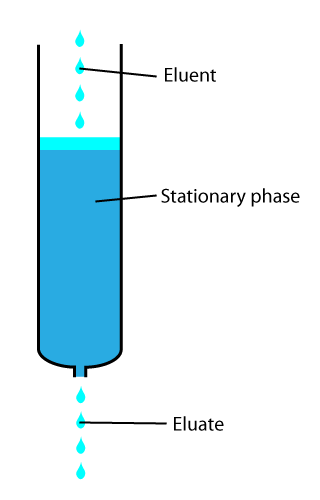
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromatography Of Chlorophyll - Step 7
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-performance Liquid Chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column. HPLC has been used for manufacturing (''e.g.'', during the production process of pharmaceutical and biological products), legal (''e.g.'', detecting performance enhancement drugs in urine), research (''e.g.'', separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (''e.g.'', detecting vitamin D levels in blood serum) purposes. Chrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thin-layer Chromatography
Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase. After the sample has been applied on the plate, a solvent or solvent mixture (known as the mobile phase) is drawn up the plate via capillary action. Because different analytes ascend the TLC plate at different rates, separation is achieved. It may be performed on the analytical scale as a means of monitoring the progress of a reaction, or on the preparative scale to purify small amounts of a compound. TLC is an analytical tool widely used because of its simplicity, relative low cost, high sensitivity, and speed of separation. TLC functions on the same principle as all chromatography: a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic lay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Analysis
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archer John Porter Martin
Archer John Porter Martin (1 March 1910 – 28 July 2002) was a British chemist who shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Richard Synge. Early life Martin's father was a GP. Martin was educated at Bedford School, and Peterhouse, Cambridge. Career Working first in the Physical Chemistry Laboratory, he moved to the Dunn Nutritional Laboratory, and in 1938 moved to Wool Industries Research Institution in Leeds. He was head of the biochemistry division of Boots Pure Drug Company from 1946 to 1948, when he joined the Medical Research Council. There, he was appointed head of the physical chemistry division of the National Institute for Medical Research in 1952, and was chemical consultant from 1956 to 1959. He specialised in biochemistry, in some aspects of vitamins E and B2, and in techniques that laid the foundation for several new types of chromatography. He developed partition chromatography whilst working on the separati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Electrochromatography
Capillary electrochromatography (CEC) is a chromatographic technique in which the mobile phase is driven through the chromatographic bed by electroosmosis. Capillary electrochromatography is a combination of two analytical techniques, high-performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis aims to separate analytes on the basis of their mass-to-charge ratio by passing a high voltage across ends of a capillary tube, which is filled with the analyte. High-performance liquid chromatography separates analytes by passing them, under high pressure, through a column filled with stationary phase. The interactions between the analytes and the stationary phase and mobile phase lead to the separation of the analytes. In capillary electrochromatography capillaries, packed with HPLC stationary phase, are subjected to a high voltage. Separation is achieved by electrophoretic migration of solutes and differential partitioning. Principle Capillary elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paper Chromatography
Paper chromatography is an analytical method used to separate coloured chemicals or substances. It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC). A paper chromatography variant, two-dimensional chromatography, involves using two solvents and rotating the paper 90° in between. This is useful for separating complex mixtures of compounds having similar polarity, for example, amino acids. The setup has three components. The mobile phase is a solution that travels up the stationary phase, due to capillary action. The mobile phase is generally a mixture of non-polar organic solvent, while the stationary phase is polar inorganic solvent water. Here paper is used to support the stationary phase, water. Polar water molecules are held inside the void space of the cellulose network of the host paper. The difference between TLC and paper chromatography is that the stationary phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eluotropic Series
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions. In a liquid chromatography experiment, for example, an analyte is generally adsorbed, or "bound to", an adsorbent in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partition Coefficient
In the physical sciences, a partition coefficient (''P'') or distribution coefficient (''D'') is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solubilities of the solute in these two liquids. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound (ionized plus un-ionized). In the chemical and pharmaceutical sciences, both phases usually are solvents. Most commonly, one of the solvents is water, while the second is hydrophobic, such as 1-octanol. Hence the partition coefficient measures how hydrophilic ("water-loving") or hydrophobic ("water-fearing") a chemical substance is. Partition coefficients are useful in estimating the distribution of drugs within the body. Hydrophobic drugs with high octanol-water partition coefficients are ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Laurence Millington Synge
Richard Laurence Millington Synge FRS FRSE FRIC FRSC MRIA (Liverpool, 28 October 1914 – Norwich, 18 August 1994) was a British biochemist, and shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Archer Martin. Life Richard Laurence Millington Synge was born in West Kirby on 28 October 1914, the son of Lawrence Millington Synge, a Liverpool stock-broker, and his wife, Katherine C. Swan. Synge was educated at the Old Hall in Wellington, Shropshire and at Winchester College. He then studied Chemistry at Trinity College, Cambridge. He spent his entire career in research, at the Wool Industries Research Association, Leeds (1941–1943), Lister Institute for Preventive Medicine, London (1943–1948), Rowett Research Institute, Aberdeen (1948–1967), and Food Research Institute, Norwich (1967–1976). It was during his time in Leeds that he worked with Archer Martin, developing partition chromatography, a technique used in the separa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |