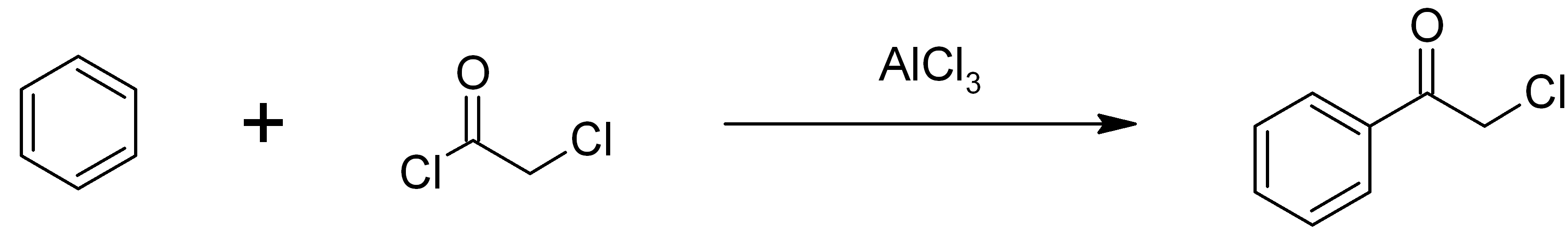|
Chemical Agent Identification Set
Chemical Agent Identification Sets (CAIS), known by several other names, were sets of glass vials or bottles that contained small amounts of chemical agents. They were employed by all branches of the United States Armed Forces from 1928-1969 for the purpose of training in detection, handling and familiarization with chemical warfare. Most CAIS were destroyed in the 1980s but the U.S. Army Chemical Materials Agency still occasionally demilitarizes CAIS that are found buried. History Nomenclature Throughout the military's use of CAIS they were known by several different common names aside from Chemical Agent Identification Sets. The other names were: Toxic Gas Sets, Chemical Agent Identification Training Sets, Instructional War Gas Identification Sets, Detonation War Gas Identification Sets, and Instructional Gas Identification Replacement Sets. General history CAIS were used by all branches of the United States military for training in detection, handling and familiarization with c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic Gas Set (CAIS) Bottle Containing Sulfur Mustard (HD)
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewisite
Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a Chemical warfare, chemical weapon, acting as a vesicant (blister agent) and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to Pelargonium, geraniums. Chemical reactions The compound is prepared by the addition of arsenic trichloride to acetylene in the presence of a suitable catalyst: :AsCl3 + C2H2 → ClCHCHAsCl2 (Lewisite) Lewisite, like other arsenous chlorides, hydrolysis, hydrolyses in water to form hydrochloric acid and chlorovinylarsenous oxide (a less-powerful blister agent): :ClCHCHAsCl2 + 2 H2O → ClCHCHAs(OH)2 + 2 HCl This reaction is accelerated in alkaline solutions, and forms acetylene and trisodium arsenate. Lewisite reacts with metals to form hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incineration
Incineration is a waste treatment process that involves the combustion of substances contained in waste materials. Industrial plants for waste incineration are commonly referred to as waste-to-energy facilities. Incineration and other high-temperature waste treatment systems are described as " thermal treatment". Incineration of waste materials converts the waste into ash, flue gas and heat. The ash is mostly formed by the inorganic constituents of the waste and may take the form of solid lumps or particulates carried by the flue gas. The flue gases must be cleaned of gaseous and particulate pollutants before they are dispersed into the atmosphere. In some cases, the heat that is generated by incineration can be used to generate electric power. Incineration with energy recovery is one of several waste-to-energy technologies such as gasification, pyrolysis and anaerobic digestion. While incineration and gasification technologies are similar in principle, the energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocky Mountain Arsenal
The Rocky Mountain Arsenal was a United States chemical weapons manufacturing center located in the Denver Metropolitan Area in Commerce City, Colorado. The site was completed December 1942, operated by the United States Army throughout the later 20th century and was controversial among local residents until its closure in 1992. Much of the site is now protected as the Rocky Mountain Arsenal National Wildlife Refuge. History After the attack on Pearl Harbor and the United States entered World War II, the U.S. Army began looking for land to create a chemical manufacturing center. Located just north of Denver, in Commerce City and close to the Stapleton Airport, the U.S. Army purchased . The location was ideal, not only because of the proximity to the airport, but because of the geographic features of the site, it was less likely to be attacked. The Rocky Mountain Arsenal manufactured chemical weapons including mustard gas, napalm, white phosphorus, lewisite, chlorine gas, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nerve Agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are acetylcholinesterase inhibitors used as poison. Poisoning by a nerve agent leads to constriction of pupils, profuse salivation, convulsions, and involuntary urination and defecation, with the first symptoms appearing in seconds after exposure. Death by asphyxiation or cardiac arrest may follow in minutes due to the loss of the body's control over respiratory and other muscles. Some nerve agents are readily vaporized or aerosolized, and the primary portal of entry into the body is the respiratory system. Nerve agents can also be absorbed through the skin, requiring that those likely to be subjected to such agents wear a full body suit in addition to a respirator. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
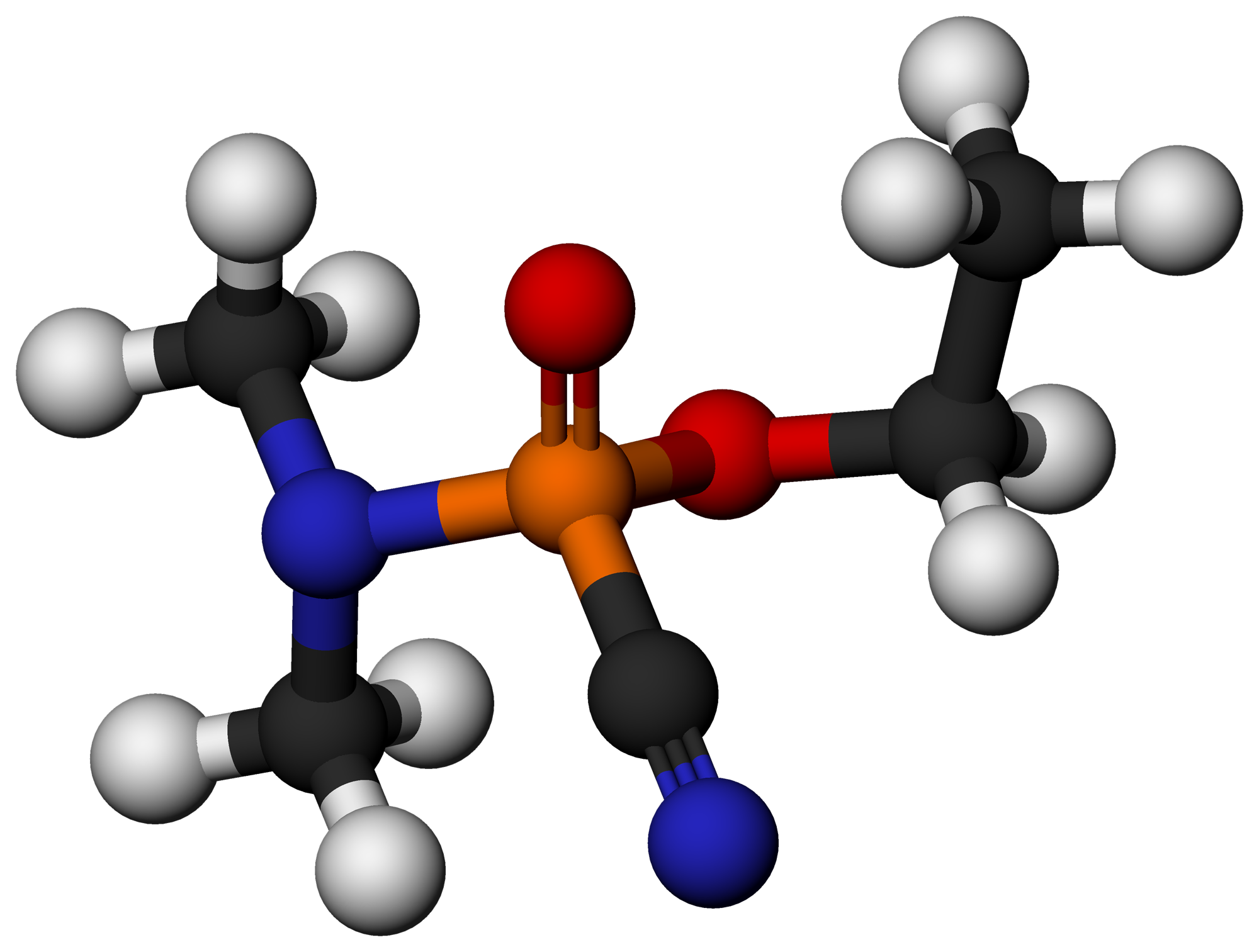
Tabun (nerve Agent)
Tabun or GA is an extremely toxic synthetic organophosphorus compound. It is a clear, colorless, and tasteless liquid with a faint fruity odor.Facts About Tabun National Terror Alert Response System It is classified as a because it fatally interferes with normal functioning of the mammalian nervous system. Its production is strictly controlled and stockpiling outlawed by the of 1993. Tabun is the first of the ''G-series'' nerve agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphosgene
Triphosgene (bis(trichloromethyl) carbonate (BTC) is a chemical compound with the formula OC(OCCl3)2. It is used as a solid substitute for phosgene, which is a gas. Triphosgene is stable up to 200 °C. Triphosgene is used in a variety of halogenation reactions. Preparation This compound is commercially available. It is prepared by exhaustive free radical chlorination of dimethyl carbonate: :CH3OCO2CH3 + 6 Cl2 → CCl3OCO2CCl3 + 6 HCl Triphosgene can be easily recrystallized from hot hexanes. Uses Triphosgene is used as a reagent in organic synthesis as a source of CO2+. It behaves like phosgene to which it cracks thermally: :OC(OCCl3)2 → 3 OCCl2 Alcohols are converted to carbonates. Primary and secondary amines are converted to ureas and isocyanates. Triphosgene has been used to synthesize organohalides. The use of triphosgene in these reactions provided a broader class of substrates that could be used for halogenation. Alkyl chlorides are synthesized via an SN2 reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloropicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2. Synthesis Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid: : HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar. Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite: : H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH or by the reaction of chloroform with nitric acid: : CHCl3 + HNO3 → CCl3NO2 + H2O Properties Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole. Pure chloropicrin is a colorless liquid, with a boiling po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Mustard
Nitrogen mustards are cytotoxic organic compounds with the chloroethylamine (Cl(CH2)2NR2) functional group. Although originally produced as chemical warfare agents, they were the first chemotherapeutic agents for treatment of cancer. Nitrogen mustards are nonspecific DNA alkylating agents. Chemical warfare During World War II nitrogen mustards were studied at the Yale School of Medicine by Alfred Gilman, Sr., Alfred Gilman and Louis Goodman, and classified human clinical trials of nitrogen mustards for the treatment of lymphoma started in December 1942. Also during World War II, an incident during the Air raid on Bari, air raid on Bari, Italy, led to the release of mustard gas that affected several hundred soldiers and civilians. Medical examination of the survivors showed a decreased number of lymphocytes. After World War II was over, the Bari incident and the Yale group's studies eventually converged prompting a search for other similar compounds. Due to its use in previous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarin
Sarin (NATO designation GB G-series, "B"">Nerve_agent#G-series.html" ;"title="hort for Nerve agent#G-series">G-series, "B" is an extremely toxic synthetic organophosphorus compound.Sarin (GB) Emergency Response Safety and Health Database. National Institute for Occupational Safety and Health. Accessed April 20, 2009. A colourless, odourless , it is used as a due to its extreme potency as a . Exposure is lethal even at very low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetophenone
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “Mace” or tear gas, its use is falling as pepper spray both works and disperses m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen Chloride
Cyanogen chloride is a highly toxic chemical compound with the formula CNCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-temperature solid that is widely used in biochemical analysis and preparation. Synthesis, basic properties, structure Cyanogen chloride is a molecule with the connectivity . Carbon and chlorine are linked by a single bond, and carbon and nitrogen by a triple bond. It is a linear molecule, as are the related cyanogen halides (NCF, NCBr, NCI). Cyanogen chloride is produced by the oxidation of sodium cyanide with chlorine. This reaction proceeds via the intermediate cyanogen (). :NaCN + Cl2 -> ClCN + NaCl The compound trimerizes in the presence of acid to the heterocycle called cyanuric chloride. Cyanogen chloride is slowly hydrolyzed by water at neutral pH to release cyanate and chloride ions: :ClCN + H2O -> NCO- + Cl- + 2H+ Applic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |