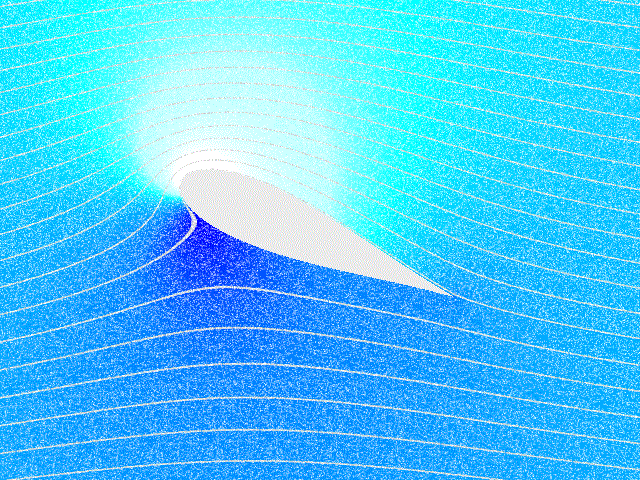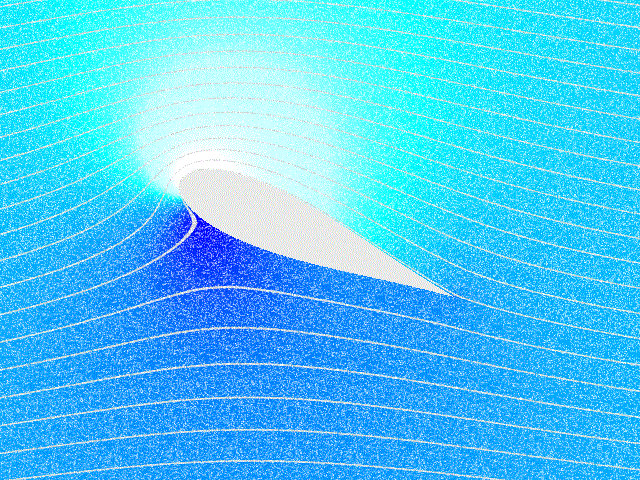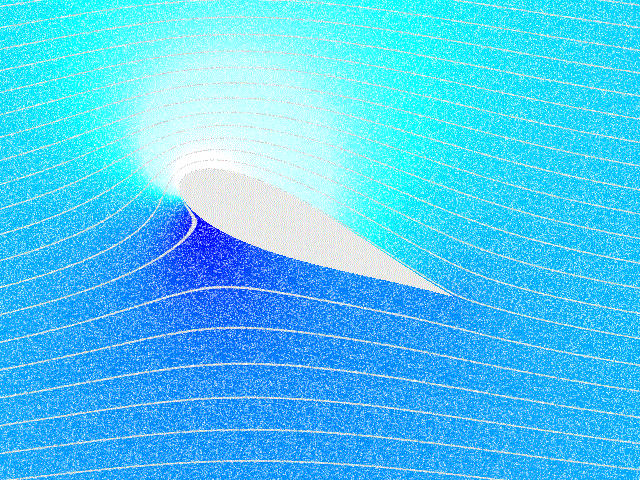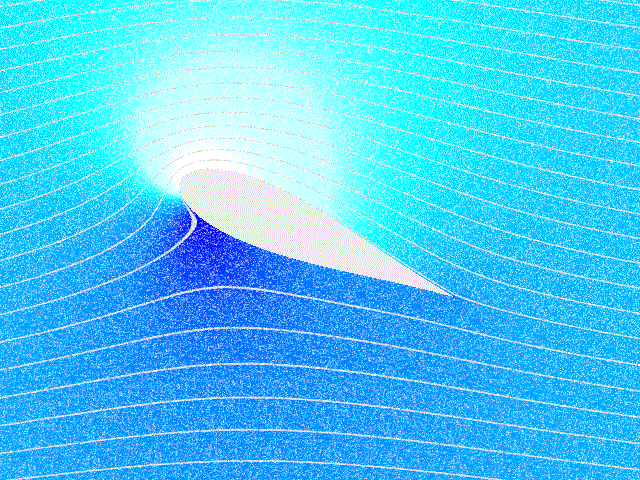
|
Cavitation In A Gear Pump
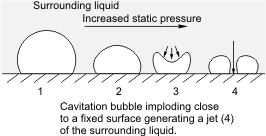
Cavitation in fluid mechanics and engineering normally is the phenomenon in which the static pressure of a liquid reduces to below the liquid's vapor pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, called "bubbles" or "voids", collapse and can generate shock waves that may damage machinery. These shock waves are strong when they are very close to the imploded bubble, but rapidly weaken as they propagate away from the implosion. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal, causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior. ''Inert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sound
In physics, sound is a vibration that propagates as an acoustic wave through a transmission medium such as a gas, liquid or solid. In human physiology and psychology, sound is the ''reception'' of such waves and their ''perception'' by the brain. Only acoustic waves that have frequency, frequencies lying between about 20 Hz and 20 kHz, the audio frequency range, elicit an auditory percept in humans. In air at atmospheric pressure, these represent sound waves with wavelengths of to . Sound waves above 20 kHz are known as ultrasound and are not audible to humans. Sound waves below 20 Hz are known as infrasound. Different animal species have varying hearing ranges, allowing some to even hear ultrasounds. Definition Sound is defined as "(a) Oscillation in pressure, stress, particle displacement, particle velocity, etc., propagated in a medium with internal forces (e.g., elastic or viscous), or the superposition of such propagated oscillation. (b) Auditory sen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturation Temperature
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry *Saturated and unsaturated compounds, a classification of compounds related to their ability to resist addition reactions ** Degree of unsaturation **Saturated fat or saturated fatty acid **Unsaturated fat or unsaturated fatty acid ** Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a concise d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor, vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the Standard atmosphere (unit), surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization. There are two main types of boiling: nucleate boiling, where small bubbles of vapour form at discrete points; and critical heat flux boiling, where the boiling surface is heated above a certain critical temperature and a film of vapour forms on the surface. Transition boiling is an intermediate, unstable form of boiling with elements of both types. The boiling point of water is 100 °C or 212 °F but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling water is used as a method of making it potable by killing Microorganism, microbes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sonoluminescence
Sonoluminescence is the emission of light from imploding bubbles in a liquid when excited by sound. Sonoluminescence was first discovered in 1934 at the University of Cologne. It occurs when a sound wave of sufficient intensity induces a gaseous cavity within a liquid to collapse quickly, emitting a burst of light. The phenomenon can be observed in stable single-bubble sonoluminescence (SBSL) and multi-bubble sonoluminescence (MBSL). In 1960, Peter Jarman proposed that sonoluminescence is thermal in origin and might arise from microshocks within collapsing cavities. Later experiments revealed that the temperature inside the bubble during SBSL could reach up to . The exact mechanism behind sonoluminescence remains unknown, with various hypotheses including hotspot, '' bremsstrahlung'', and collision-induced radiation. Some researchers have even speculated that temperatures in sonoluminescing systems could reach millions of kelvins, potentially causing thermonuclear fusion; this i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor
In physics, a vapor (American English) or vapour (Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensation, condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist at any pressure. In the Earth's atmosphere, atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercavitation
Supercavitation is the phenomenon of a cavitation bubble reducing skin friction drag on a submerged object and enabling high speeds. Applications include torpedoes and propellers, but in theory, the technique could be extended to an entire underwater vessel. Physical principle Cavitation is the formation of vapour bubbles in liquid caused by flow around an object. Bubbles form when water accelerates around sharp corners and the pressure drops below the vapour pressure. Pressure increases upon deceleration, and the water generally reabsorbs the vapour; however, vapour bubbles can implode and apply small concentrated impulses that may damage surfaces like ship propellers and pump impellers. The potential for vapour bubbles to form in a liquid is given by the nondimensional cavitation number. It equals local pressure minus vapour pressure, divided by dynamic pressure. At increasing depths (or pressures in piping), the potential for cavitation is lower because the difference ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluid Dynamics
In physics, physical chemistry and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids – liquids and gases. It has several subdisciplines, including (the study of air and other gases in motion) and (the study of water and other liquids in motion). Fluid dynamics has a wide range of applications, including calculating forces and moment (physics), moments on aircraft, determining the mass flow rate of petroleum through pipeline transport, pipelines, weather forecasting, predicting weather patterns, understanding nebulae in interstellar space, understanding large scale Geophysical fluid dynamics, geophysical flows involving oceans/atmosphere and Nuclear weapon design, modelling fission weapon detonation. Fluid dynamics offers a systematic structure—which underlies these practical disciplines—that embraces empirical and semi-empirical laws derived from flow measurement and used to solve practical problems. The solution to a fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogenize
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities. Etymology and spelling The words ''homogeneous'' and ''heterogeneous'' come from Medieval Latin ''homogeneus'' and ''heterogeneus'', from Ancient Greek ὁμογενής (''homogenēs'') and ἑτερογενής (''heterogenēs''), from ὁμός (''homos'', "same") and ἕτερος (''heteros'', "other, another, different") respectively, followed by γένος (''genos'', "kind"); -ous is an adjectival suffix. Alternate spellings omitting the last ''-e-'' (and the associated pronunciations) are common, but mistaken: ''homogenous'' is strictly a biological/pathological term which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |