|
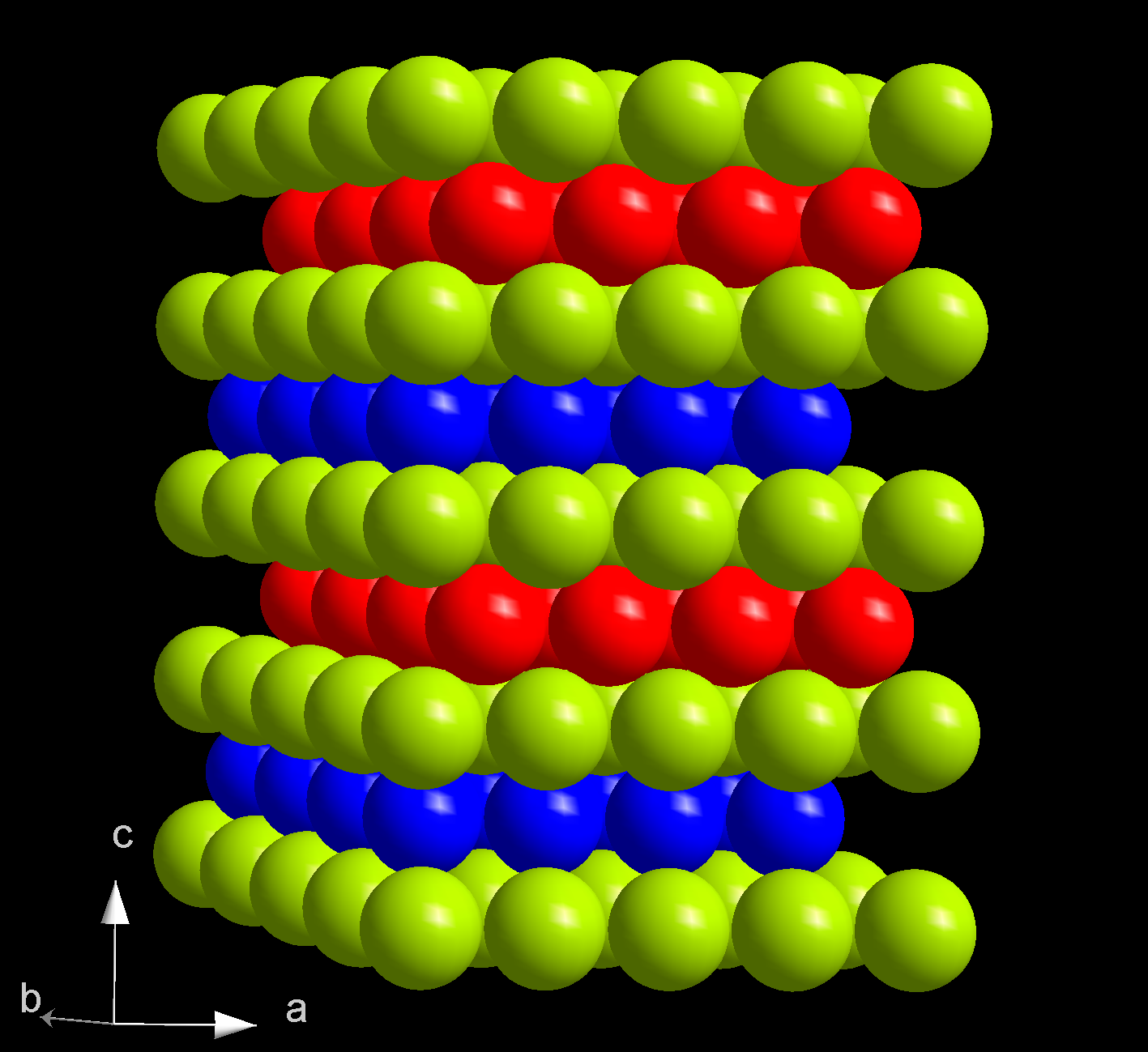
Californium Tribromide
Californium(III) bromide is an inorganic compound, a salt with a chemical formula CfBr3. Like in californium(III) oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3), californium(III) chloride, and californium(III) iodide (CfI3), the californium atom has an oxidation state of +3. Properties Californium(III) bromide is shown to crystallize in both the AlCl3 and FeCl3 type structures. In the former structure, the californium ion is six coordinated and the three independent Cf-Br bond lengths are 279.5±0.9 pm, 282.7±1.1 pm, and 282.8±0.8 pm. Californium(III) bromide partially decomposes into californium(II) bromide under high temperature. :2 CfBr3 ->Delta T2 CfBr2 + Br2 In the radioactive decay of berkelium-249 to californium-249, the oxidation number and crystal structure are preserved. The six-coordinate berkelium(III) bromide (AlCl3-type monoclinic structure) decays to produce a six-coordinate californium(III) bromide, whereas an eight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(III) Oxide
Californium(III) oxide is a binary inorganic compound of californium and oxygen Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ... with the formula . It is one of the first obtained solid compounds of californium, synthesized in 1958. __TOC__ Synthesis The compound can be prepared by burning ionite in air, on which ions of trivalent californium are sorbed, at a temperature of 1400 °C. It can also be obtained by β-decay of berkelium(III) oxide. Physical properties Californium(III) oxide forms a yellow-green solid with a melting point of 1750 °C and exists in three modifications. The body-centered cubic modification forms a crystal lattice with a = 1083.9 ± 0.4 pm. The transition temperature between body-centered cubic and monoclinic structures is about 1400 °C. It is insoluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(III) Chloride
Californium(III) chloride is an inorganic compound with a chemical formula CfCl3. As in californium(III) oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3) and iodide (CfI3), the californium atom has an oxidation state of +3. Preparation Californium(III) chloride can prepared by reacting californium(III) oxide with hydrogen chloride. :Cf2O3 + 6 HCl → 2 CfCl3 + 3 H2O Properties Chemical properties When heating californium(III) chloride until 500 °C, it can hydrolyse to produce californium oxychloride. Physical properties Californium(III) chloride is soluble in water, giving Cf3+ and Cl− ions. This salt has an emerald-green color. Its crystal structure is hexagonal. It is strongly radioactive. See also * Californium * Californium compounds Californium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geomet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals. Electronic and optical properties All forms of ferric chloride are paramagnetic, owing to the presence of unpaired electrons residing in 3d orbitals. Although Fe(III) chloride can be octahedral or tetrahedral (or both, see structure section), all of these forms have five unpaired electrons, one per d-orbital. The high spin d5 electronic configuration requires that d-d electronic transitions are spin forbidden, in addition to violating the Laporte rule. This double forbidden-ness results in its solutions being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The longest-lived and second-most important isotope, 247Bk, can be synthesized via irradiation of 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical application of berkeli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium(III) Bromide
Plutonium(III) bromide is an inorganic salt of bromine and plutonium with the formula PuBr3. This radioactive green solid has few uses, however its crystal structure is often used as a structural archetype in crystallography. Crystal structure The PuBr3 crystal structure was first published in 1948 by William Houlder Zachariasen. The compound forms orthorhombic crystals, a type of square antiprism, within which the Pu atoms adopt an 8-coordinate bicapped trigonal prismatic arrangement. Its Pearson symbol is oS16 with the corresponding space group No. 63 (in International Union of Crystallography classification) or Cmcm (in Hermann–Mauguin notation). The majority of trivalent chloride and bromide salts of lanthanide and actinides crystallise in the PuBr3 structure. References Plutonium(III) compounds Actinide halides Crystal structure types {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |