|
CPCCOEt
CPCCOEt is a drug used in scientific research, which acts as a non-competitive antagonist at the metabotropic glutamate receptor subtype mGluR1, with high selectivity although only moderate binding affinity. It is used mainly in basic research into the function of the mGluR1 receptor, including the study of behavioural effects in animals including effects on memory and addiction. See also * PHCCC PHCCC is a research drug which acts as a glutamate receptor ligand, particularly being a positive allosteric modulator at the mGluR4 subtype, as well as an agonist at mGluR6. It has anxiolytic effects in animal studies. PHCCC and similar drugs h ... References MGlu1 receptor antagonists Benzopyrans Ethyl esters Cyclopropanes Ketoximes {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PHCCC
PHCCC is a research drug which acts as a glutamate receptor ligand, particularly being a positive allosteric modulator at the mGluR4 subtype, as well as an agonist at mGluR6. It has anxiolytic effects in animal studies. PHCCC and similar drugs have been suggested as novel treatments for Parkinson's disease Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms becom .... See also * CPCCOEt References Cyclopropanes MGlu4 receptor agonists Ketoximes Anilides {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
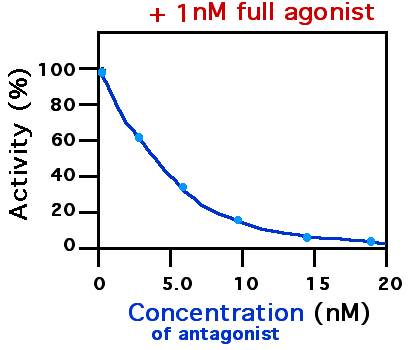
Antagonist (pharmacology)
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Metabotropic Glutamate Receptor
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter. Function and structure The mGluRs perform a variety of functions in the central and peripheral nervous systems: For example, they are involved in learning, memory, anxiety, and the perception of pain. They are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues. Like other metabotropic receptors, mGluRs have seven transmembrane domains that span the cell membrane. Unlike ionotropic receptors, metabotropic glutamate receptors are not ion channels. Instead, they activate biochemical cascades, leading to the modification ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabotropic Glutamate Receptor 1
The glutamate receptor, metabotropic 1, also known as GRM1, is a human gene which encodes the metabotropic glutamate receptor 1 (mGluR1) protein. Function L-glutamate is the major excitatory neurotransmitter in the central nervous system and activates both ionotropic and metabotropic glutamate receptors. Glutamatergic neurotransmission is involved in most aspects of normal brain function and can be perturbed in many neuropathologic conditions. The metabotropic glutamate receptors are a family of G protein-coupled receptors, that have been divided into 3 groups on the basis of sequence homology, putative signal transduction mechanisms, and pharmacologic properties. Group I, which includes GRM1 alongside GRM5, have been shown to activate phospholipase C. Group II includes GRM2 and GRM3 while Group III includes GRM4, GRM6, GRM7 and GRM8. Group II and III receptors are linked to the inhibition of the cyclic AMP cascade but differ in their agonist selectivities. Alte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzopyrans
Benzopyran is a polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring. According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2''H''-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues. There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature. Some benzopyrans have shown anticancerous activity ''in vitro''. The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Esters
{{disambiguation ...
Ethyl may refer to: Arts and entertainment * Cold Ethyl, a Swedish rock band * Ethyl Sinclair, a character in the ''Dinosaurs'' television show Science and technology * Ethyl group, an organic chemistry moiety * Ethyl alcohol (or ethanol) * Ethyl Corporation, a fuel additive company ** Tetraethyllead-treated gasoline See also * Ethel (other) Ethel is an English name, usually used as a feminine given name, also a surname. Ethel or ETHEL may also refer to: Places United States * Ethel, Arkansas, an unincorporated community * Ethel, Indiana, an unincorporated community * Ethel, Missi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into clin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |