|
CHK-336
CHK-336 is a small molecule lactate dehydrogenase inhibitor developed by Chinook Therapeutics (a Novartis Company). CHK-336 is a first-in-class, orally available, and liver-targeted molecule and is being investigated for the treatment of primary hyperoxaluria. By inhibiting the final and only committed step in hepatic oxalate synthesis, CHK-336 could in principle treat all forms of primary hyperoxaluria. In April 2022, a phase 1 clinical trial of CHK-336 was initiated. This trial was designed to evaluate the safety, tolerability, and pharmacokinetic profile of CHK-336 in healthy volunteers. CHK-336 was found to be generally well-tolerated in single doses up to 500 mg and multiple doses up to 60 mg for 14 days. Pharmacokinetic evaluation supported once-daily dosing, and use of a 13C2-Glycolic acid, glycolate tracer established proof-of-mechanism that CHK-336 blocks hepatic oxalate production. This clinical trial was paused in April 2023 upon one serious adverse event of ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CHK-336 Correct Label 8FW6
CHK-336 is a small molecule lactate dehydrogenase inhibitor developed by Chinook Therapeutics (a Novartis Company). CHK-336 is a first-in-class, orally available, and liver-targeted molecule and is being investigated for the treatment of primary hyperoxaluria. By inhibiting the final and only committed step in hepatic oxalate synthesis, CHK-336 could in principle treat all forms of primary hyperoxaluria. In April 2022, a phase 1 clinical trial of CHK-336 was initiated. This trial was designed to evaluate the safety, tolerability, and pharmacokinetic profile of CHK-336 in healthy volunteers. CHK-336 was found to be generally well-tolerated in single doses up to 500 mg and multiple doses up to 60 mg for 14 days. Pharmacokinetic evaluation supported once-daily dosing, and use of a 13C2-glycolate tracer established proof-of-mechanism that CHK-336 blocks hepatic oxalate production. This clinical trial was paused in April 2023 upon one serious adverse event of anaphylaxis in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Target-mediated Drug Disposition
Target-mediated drug disposition (TMDD) is the process in which a drug binds with high affinity to its pharmacological target (for example, a receptor) to such an extent that this affects its pharmacokinetic characteristics. Various drug classes can exhibit TMDD, most often these are large compounds (biologics such as antibodies, cytokines or growth factors) but also smaller compounds can exhibit TMDD (such as warfarin and CHK-336 CHK-336 is a small molecule lactate dehydrogenase inhibitor developed by Chinook Therapeutics (a Novartis Company). CHK-336 is a first-in-class, orally available, and liver-targeted molecule and is being investigated for the treatment of primary hy ...). A typical TMDD pattern of antibodies displays non-linear clearance and can be seen at concentration ranges that are usually defined as 'mid-to-low'. In this concentration range, the target is partly saturated. References {{pharmacology-stub Pharmacokinetics Medicinal chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperoxaluria
Hyperoxaluria is an excessive urinary excretion of oxalate. Individuals with hyperoxaluria often have calcium oxalate kidney stones. It is sometimes called Bird's disease, after Golding Bird, who first described the condition. Presentation Causes Hyperoxaluria can be primary (as a result of a genetic defect) or secondary to another disease process. Type I primary hyperoxaluria (PH1) is associated mutations in the gene encoding AGXT, a key enzyme involved in oxalate metabolism. PH1 is an example of a protein mistargeting disease, wherein AGXT shows a trafficking defect. Instead of being trafficked to peroxisomes, it is targeted to mitochondria, where it is metabolically deficient despite being catalytically active. Type II is associated with GRHPR. Secondary hyperoxaluria can occur as a complication of jejunoileal bypass, or in a patient who has lost much of the ileum with an intact colon. In these cases, hyperoxaluria is caused by excessive gastrointestinal oxalate absorpti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactate Dehydrogenase A
Lactate dehydrogenase A (LDHA) is an enzyme which in humans is encoded by the ''LDHA'' gene. It is a monomer of lactate dehydrogenase, which exists as a tetramer. The other main subunit is lactate dehydrogenase B (LDHB). Function Lactate dehydrogenase A catalyzes the inter-conversion of pyruvate and L- lactate with concomitant inter-conversion of NADH and NAD+. LDHA is found in most somatic tissues, though predominantly in muscle tissue and tumors, and belongs to the lactate dehydrogenase family. It has long been known that many human cancers have higher LDHA levels compared to normal tissues. It has also been shown that LDHA plays an important role in the development, invasion and metastasis of malignancies. Mutations in LDHA have been linked to exertional myoglobinuria. Interactive pathway map LDHA Inhibitors The following compounds have been demonstrated to inhibit the LDHA enzyme: * Oxamate * Epigallocatechin gallate * Quinoline 3-sulfonamide * CHK-336 CHK-336 is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactate Dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of pyruvic acid, pyruvate to lactic acid, lactate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. LDH exists in four distinct enzyme classes. This article is specifically about the NAD(P)-dependent L-lactate dehydrogenase. Other LDHs act on D-lactate and/or are dependent on cytochrome c: D-lactate dehydrogenase (cytochrome) and L-lactate dehydrogenase (cytochrome). LDH is expressed extensively in body tissues, such as blood cells and heart muscle. Because it is released during tissue damage, it is a marker of common injuries and disease such as heart failure. Reaction Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactic acid, lactate with concomitant interconversion of NADH and Nicotinamide adenine dinucleotide, NAD+. It converts pyruvate, the final produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
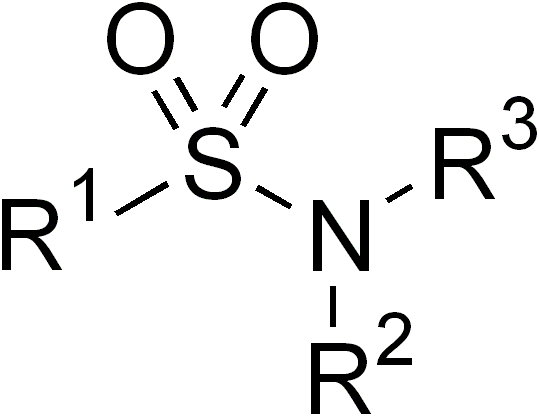
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane. Preparation Pyrazoles are syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazoles
Thiazole (), or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group when part of a larger molecule. Being planar thiazoles are characterized by significant pi-electron delocalization and have some degree of aromaticity, more so than the corresponding oxazoles. This aromaticity is evidenced by the 1H NMR chemical shift of the ring protons, which absorb between 7.27 and 8.77 ppm, indicating a strong diamagnetic ring current. The calculated pi-electron density marks C5 as the primary site for electrophilic substitution, and C2-H a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRHPR
Glyoxylate reductase/hydroxypyruvate reductase is an enzyme that in humans is encoded by the ''GRHPR'' gene. This gene encodes an enzyme with hydroxypyruvate reductase, glyoxylate reductase, and D- glycerate dehydrogenase enzymatic activities. The enzyme has widespread tissue expression and has a role in metabolism. Type II hyperoxaluria Hyperoxaluria is an excessive urinary excretion of oxalate. Individuals with hyperoxaluria often have calcium oxalate kidney stones. It is sometimes called Bird's disease, after Golding Bird, who first described the condition. Presentation Caus ... is caused by mutations in this gene. GRHPR mutation analysis needs to pay attention to primer design, because allele dropout can cause false-positive result. References External links GeneReviews/NCBI/NIH/UW entry on Primary Hyperoxaluria Type 2 Further reading * * * * * * * * * * * * {{gene-9-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AGXT
Serine—pyruvate aminotransferase is an enzyme that in humans is encoded by the ''AGXT'' gene. This gene is expressed only in the liver and the encoded protein is localized mostly in the peroxisomes, where it is involved in glyoxylate detoxification. Mutations in this gene, some of which alter subcellular targeting, have been associated with type I primary hyperoxaluria Hyperoxaluria is an excessive urinary excretion of oxalate. Individuals with hyperoxaluria often have calcium oxalate kidney stones. It is sometimes called Bird's disease, after Golding Bird, who first described the condition. Presentation Caus .... See also * Peroxisomal disorder References External links GeneReviews/NIH/NCBI/UW entry on Primary Hyperoxaluria Type 1* * Further reading * * * * * * * * * * * * * * * * * {{gene-2-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organo Anion Transporter Family
Members of the Organic Anion Transporter (OAT) Family (organic-anion-transporting polypeptides, OATP) are membrane transport proteins or 'transporters' that mediate the transport of mainly organic anions across the cell membrane. Therefore, OATPs are present in the lipid bilayer of the cell membrane, acting as the cell's gatekeepers. OATPs belong to the Solute Carrier Family (SLC) and the major facilitator superfamily. The generalized transport reactions catalyzed by members of the OAT family are: Anion (in) → Anion (out) Anion1 (in) + Anion2 (out) → Anion1 (out) + Anion2 (in) Function Proteins of the OAT family catalyze the Na+-independent facilitated transport of fairly large amphipathic organic anions (and less frequently neutral or cationic drugs), such as bromosulfobromophthalein, prostaglandins, conjugated and unconjugated bile acids (taurocholate and cholate), steroid conjugates, thyroid hormones, anionic oligopeptides, drugs, toxins and other xenobiotics. One famil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |