|
CFC-112a
Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four chlorine atoms and two fluorine atoms. With a boiling point of 91.5°C it is the freon with second highest boiling point. Tetrachlorodifluoroethane as made is a mixture of the symmetrical and asymmetric isomers. Preparation Tetrachloro-1,1-difluoroethane can be prepared in 40% yield by reacting 1,1,2-trichloro-1,2,2-trifluoroethane (freon 113) with aluminium chloride at 60°C. It can also be made in a reaction with hydrogen fluoride with hexachloroethane or tetrachloroethane with extra chlorine. This reaction occurs with an aluminium fluoride catalyst at 400°C. unsymmetrical trichlorotrifluoroethane (CCl2FCClF2) is also produced along with other chlorofluorocarbons. Separation of the symmetrical and unsymmetrical isomer is difficult. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CFC-112
Tetrachloro-1,2-difluoroethane is a chlorofluorocarbon known as Freon 112, CFC-112 or R-112. It has a symmetrical structure CCl2FCCl2F and so can be called symmetrical tetrachlorodifluoroethane. "Symmetrical" may also be abbreviated to "s-" or "sym-". In contrast an asymmetrical isomer has formula CCl3CClF2. Production CFC-112 can be made in a reaction of hexachloroethane or tetrachloroethane with hydrogen fluoride and extra chlorine. This reaction is catalysed by aluminium fluoride with some extra iron, nickel and chromium at 400°C. The extra metal in the catalyst yields can be 98% compared with the unsymmetrical isomer. Mixed with perfluorooctane, it is a solvent for polydimethylsiloxane. CFC-112 can be prepared as a mixture with other hydrochlorofluorocarbons from trichloroethylene and anhydrous hydrogen fluoride when electric current is passed through. When CFC-11 is packaged with alcohol in a metal container, a free radical reaction can result in production of CFC-112. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbons
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahedral symmetry. Because the fluorine and chlorine atoms diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
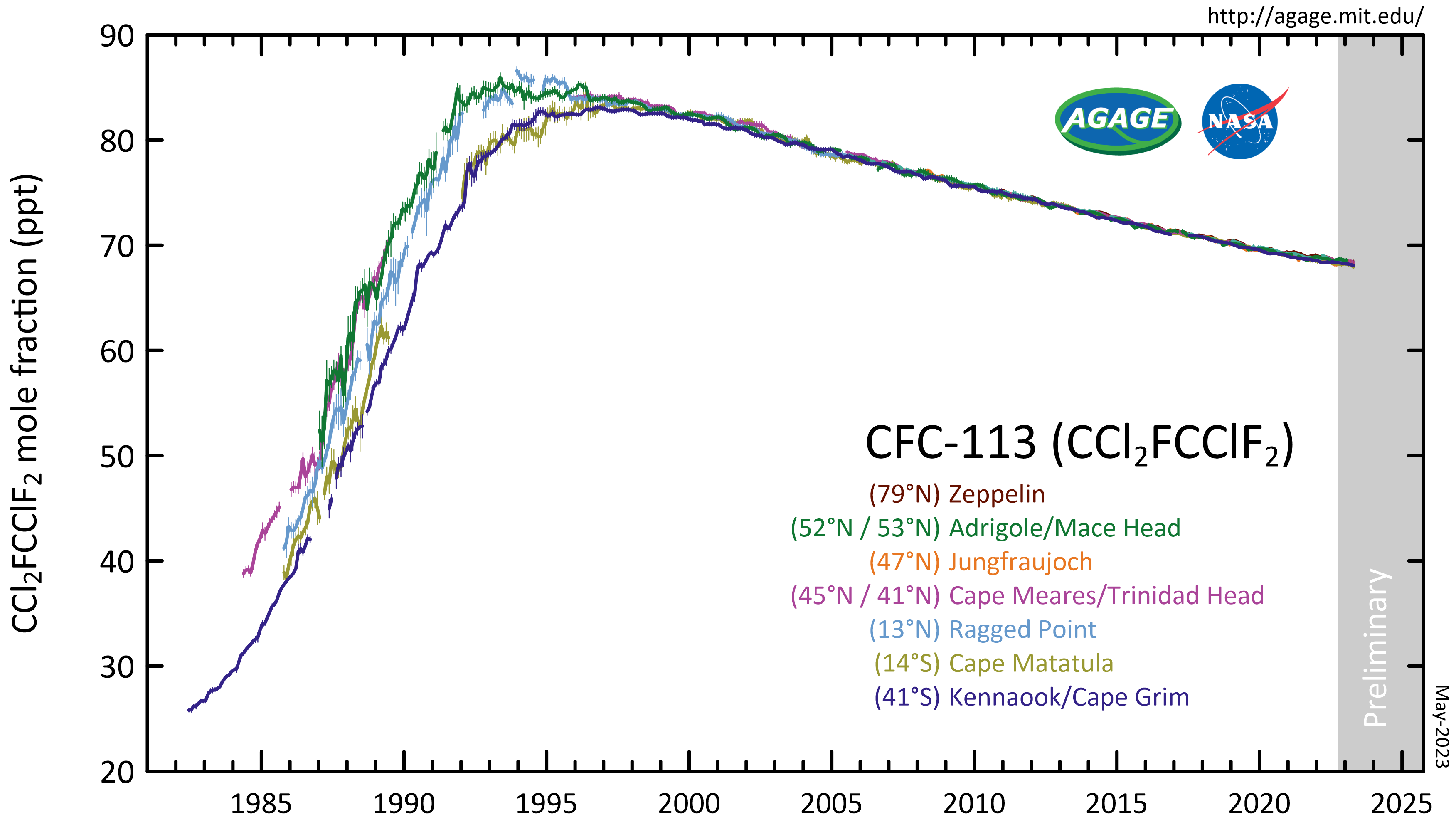
1,1,2-trichloro-1,2,2-trifluoroethane
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachloroethane
Hexachloroethane (perchloroethane) is an organochlorine compound with the chemical formula . Its structure is . It is a white or colorless solid at room temperature with a camphor-like odor. It has been used by the military in smoke compositions, such as base-eject smoke munitions ( smoke grenades). Hexachloroethane was discovered along with carbon tetrachloride by Michael Faraday in 1820. Faraday obtained it by chlorinating ethylene. Manufacture Chlorination of tetrachloroethylene at 100–140 °C with the presence of iron(III) chloride is the most commonly used commercial production method, however several other methods exist. A high purity form can be produced in a small scale by reacting chlorine together with barium carbide. In September 1997, it was reported as no longer being produced in the United States for commercial distribution, but was produced as a by-product of industrial chlorination process. : Applications Hexachloroethane has been used in the formulation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethane
{{Chemistry index ...
Tetrachloroethane () may refer to either of two isomeric chemical compounds: * 1,1,1,2-Tetrachloroethane (R-130a) * 1,1,2,2-Tetrachloroethane (R-130) See also * Tetrachloroethene * Trichloroethane * Tetrafluoroethane * Tetrabromoethane 1,1,2,2-Tetrabromoethane, or simply tetrabromoethane (TBE), is a halogenated hydrocarbon, chemical formula C2H2Br4. Although three bromine atoms may bind to one of the carbon atoms creating 1,1,1,2-tetrabromoethane this is not thermodynamically fav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,2-Trichloro-1,2,2-trifluoroethane
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorodifluoroethylene
A dichlorodifluoroethylene (systematically named dichlorodifluoroethene) is one of three compounds with the chemical formula . Dichlorodifluoroethylenes are colourless gases, and are some of the simplest chlorodifluoroalkenes. The structural isomers are used as intermediates or precursors in the production of other industrial chemicals. 1,1-Dichloro-2,2-difluoroethylene 1,1-Dichloro-2,2-difluoroethylene is a low-boiling liquid that is used a refrigerant. It may also be used as a solvent, but has practical limitations as such, because of its low boiling point (commercial listings, 19 °C; lit. 17 °C). It is regarded as a hazardous chemical for being toxic by inhalation (see MSDS), and a low-boiling liquid, and it causes irritation when it comes into contact with the skin and mucous membranes. Its ASHRAE The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, vent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fasciola Hepatica
''Fasciola hepatica'', also known as the common liver fluke or sheep liver fluke, is a parasitism, parasitic trematode (fluke (flatworm), fluke or flatworm, a type of helminth) of the class (biology), class Trematoda, phylum Platyhelminthes. It infects the livers of various mammals, including humans, and is transmitted by sheep and cattle to humans all over the world. The disease caused by the fluke (flatworm), fluke is called fasciolosis or fascioliasis, which is a type of helminthiasis and has been classified as a neglected tropical disease. Fasciolosis is currently classified as a plant/food-borne trematode infection, often acquired through eating the parasite's Trematode life cycle stages, metacercariae encysted on plants. ''F. hepatica'', which is distributed worldwide, has been known as an important parasite of sheep and cattle for decades and causes significant economic losses in these livestock species, up to £23'' ''million in the UK alone. Because of its relat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |