|
CFC-112
Tetrachloro-1,2-difluoroethane is a chlorofluorocarbon known as Freon 112, CFC-112 or R-112. It has a symmetrical structure CCl2FCCl2F and so can be called symmetrical tetrachlorodifluoroethane. "Symmetrical" may also be abbreviated to "s-" or "sym-". In contrast an asymmetrical isomer has formula CCl3CClF2. Production CFC-112 can be made in a reaction of hexachloroethane or tetrachloroethane with hydrogen fluoride and extra chlorine. This reaction is catalysed by aluminium fluoride with some extra iron, nickel and chromium at 400°C. The extra metal in the catalyst yields can be 98% compared with the unsymmetrical isomer. Mixed with perfluorooctane, it is a solvent for polydimethylsiloxane. CFC-112 can be prepared as a mixture with other hydrochlorofluorocarbons from trichloroethylene and anhydrous hydrogen fluoride when electric current is passed through. When CFC-11 is packaged with alcohol in a metal container, a free radical reaction can result in production of CFC-112. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CFC-112a
Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four chlorine atoms and two fluorine atoms. With a boiling point of 91.5°C it is the freon with second highest boiling point. Tetrachlorodifluoroethane as made is a mixture of the symmetrical and asymmetric isomers. Preparation Tetrachloro-1,1-difluoroethane can be prepared in 40% yield by reacting 1,1,2-trichloro-1,2,2-trifluoroethane (freon 113) with aluminium chloride at 60°C. It can also be made in a reaction with hydrogen fluoride with hexachloroethane or tetrachloroethane with extra chlorine. This reaction occurs with an aluminium fluoride catalyst at 400°C. unsymmetrical trichlorotrifluoroethane (CCl2FCClF2) is also produced along with other chlorofluorocarbons. Separation of the symmetrical and unsymmetrical isomer is difficult. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachlorodifluoroethane
Tetrachlorodifluoroethane is the name for two isomers. * Tetrachloro-1,2-difluoroethane, also known as CFC-112 * Tetrachloro-1,1-difluoroethane Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four c ..., also known as CFC-112a {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbons
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahedral symmetry. Because the fluorine and chlorine atoms diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and 2,3,3,3-Tetrafluoropropene, R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahedral symmetry. Because the fluorine and chlorine atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
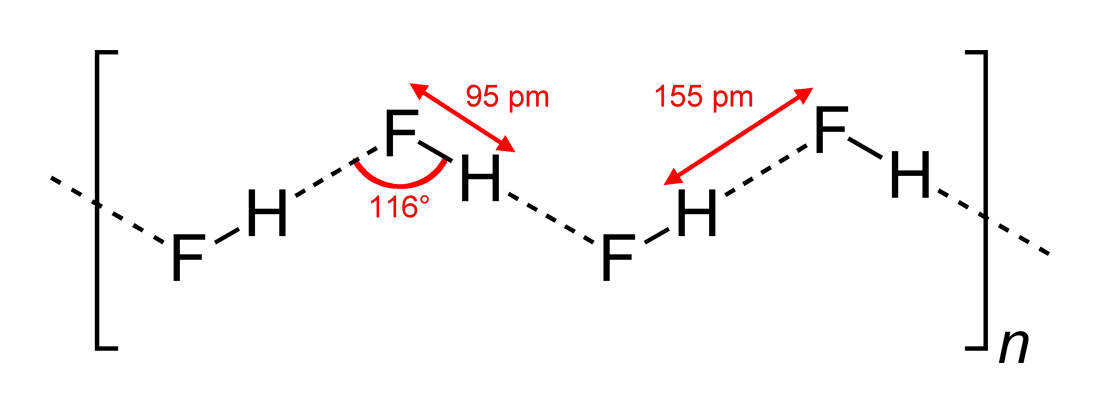
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor. Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins. Notably, it is not miscible with salt solutions and can be separated by adding sodium chloride in a process known as salting out. It forms an azeotrope with water, resulting in a boiling point of 80.37 °C and is characterized by its slightly bitter taste. Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm. Chemically, it can be oxidized to acetone or undergo various reactions to form compounds like isopropoxides or aluminium isopropoxide. As an isopropyl group linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fasciola Hepatica
''Fasciola hepatica'', also known as the common liver fluke or sheep liver fluke, is a parasitism, parasitic trematode (fluke (flatworm), fluke or flatworm, a type of helminth) of the class (biology), class Trematoda, phylum Platyhelminthes. It infects the livers of various mammals, including humans, and is transmitted by sheep and cattle to humans all over the world. The disease caused by the fluke (flatworm), fluke is called fasciolosis or fascioliasis, which is a type of helminthiasis and has been classified as a neglected tropical disease. Fasciolosis is currently classified as a plant/food-borne trematode infection, often acquired through eating the parasite's Trematode life cycle stages, metacercariae encysted on plants. ''F. hepatica'', which is distributed worldwide, has been known as an important parasite of sheep and cattle for decades and causes significant economic losses in these livestock species, up to £23'' ''million in the UK alone. Because of its relat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling. PDMS is particularly known for its unusual rheological (or flow) properties. It is optically clear and, in general, inert, non-toxic, and non-flammable. It is one of several types of silicone oil (polymerized siloxane). The applications of PDMS range from contact lenses and medical devices to elastomers; it is also present in shampoos (as it makes hair shiny and slippery), food ( antifoaming agent), caulk, lubricants and heat-resistant tiles. Structure The chemical formula of PDMS is , where ''n'' is the number of repeating monomer units. Industrial synthesis can begin from dimethyldichlorosilane and water by the following net reaction: : + (''n''+1) The polymerization reaction evolves hydrochloric acid. For medical and domestic applications, a process wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethylene
Trichloroethylene (TCE) is an organochloride with the formula C2HCl3, commonly used as an industrial metal-degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste.Trichloroethylene (TCE) on ATSDR Its name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |