|
C2orf74
C2orf74, also known as LOC339804, is a protein encoding gene located on the short arm of chromosome 2 near position 15 (2p15). Isoform 1 of the gene is 19,713 base pairs long. C2orf74 has orthologs in 135 different species, including primarily placental mammals and some marsupials. The protein encoded by the C2orf74 gene has two isoforms, the longest of which (isoform 1) is 187 amino acids in length. This protein is linked to the development of autoimmune disorders such as ankylosing spondylitis and diseases affecting the colon Gene C2orf74 is a gene located on the plus strand at 2p15 in humans. It is 19,713 base pairs in length beginning at 61,145,116 and ending at 61,164,828 and includes 8 exons. Other genes within its neighborhood include KIAA841, LOC105374759, LOC105374758, LOC339803, AHSA2P, USP34, and SNORA70B. Transcripts Transcript variants C2orf74 has 6 validated mRNA products created via alternative splicing that give rise to two different isoforms. An exte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C2orf74 Location
C, or c, is the third Letter (alphabet), letter in the Latin alphabet, used in the English alphabet, modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is English alphabet#Letter names, ''cee'' (pronounced ), plural ''cees''. History "C" comes from the same letter as "G". The Semitic people, Semites named it gimel. The sign is possibly adapted from an Egyptian hieroglyphs, Egyptian hieroglyph for a Staff-sling, staff sling, which may have been the meaning of the name ''gimel''. Another possibility is that it depicted a camel, the Semitic name for which was ''gamal''. Barry B. Powell, a specialist in the history of writing, states "It is hard to imagine how gimel = "camel" can be derived from the picture of a camel (it may show his hump, or his head and neck!)". In the Etruscan language, plosive consonants had no contrastive phonation, voicing, so the Greek language, Greek 'Gamma, Γ' (Gamma) was adopted into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research.Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function. Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG-box
In molecular biology, the HMG-box (high mobility group box) is a protein domain which is involved in DNA binding. Structure The structure of the HMG-box domain contains three alpha helices separated by loops (see figure to the right). Function HMG-box containing proteins only bind non- B-type DNA conformations (kinked or unwound) with high affinity. HMG-box domains are found in some high mobility group proteins, which are involved in the regulation of DNA-dependent processes such as transcription, replication, and DNA repair, all of which require changing the conformation of chromatin Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important .... The single and the double box HMG proteins alter DNA architecture by inducing bends upon binding.D. Murugesapillai ''et al''Single-molecule stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromodomain
A bromodomain is an approximately 110 amino acid protein domain that recognizes acetylated lysine residues, such as those on the ''N''-terminal tails of histones. Bromodomains, as the "readers" of lysine acetylation, are responsible in transducing the signal carried by acetylated lysine residues and translating it into various normal or abnormal phenotypes. Their affinity is higher for regions where multiple acetylation sites exist in proximity. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices each separated by loop regions of variable lengths that form a hydrophobic pocket that recognizes the acetyl lysine. Discovery The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the drosophila gene ''Brahma''/''brm'', and showed sequence similarity to genes involved in transcriptional activation. The name "brom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fork Head Domain
The fork head domain is a type of protein domain that is often found in transcription factors and whose purpose is to bind DNA. Function The fork head protein of ''Drosophila melanogaster'', a transcription factor that promotes terminal rather than segmental development, contains neither homeodomains nor zinc-fingers characteristic of other transcription factors. Instead, it contains a distinct type of DNA-binding region, containing around 100 amino acids, which has since been identified in a number of transcription factors (including ''D. melanogaster'' FD1-5, mammalian HNF3, human HTLF, ''Saccharomyces cerevisiae'' HCM1, etc.). This is referred to as the fork head domain but is also known as a "winged helix". The fork head domain binds B-DNA as a monomer, but shows no similarity to previously identified DNA-binding motifs. Although the domain is found in several different transcription factors, a common function is their involvement in early developmental decisions of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C2orf74 Exon-promoter Map
C, or c, is the third letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''cee'' (pronounced ), plural ''cees''. History "C" comes from the same letter as "G". The Semites named it gimel. The sign is possibly adapted from an Egyptian hieroglyph for a staff sling, which may have been the meaning of the name ''gimel''. Another possibility is that it depicted a camel, the Semitic name for which was ''gamal''. Barry B. Powell, a specialist in the history of writing, states "It is hard to imagine how gimel = "camel" can be derived from the picture of a camel (it may show his hump, or his head and neck!)". In the Etruscan language, plosive consonants had no contrastive voicing, so the Greek ' Γ' (Gamma) was adopted into the Etruscan alphabet to represent . Already in the Western Greek alphabet, Gamma first took a '' form in Early Etruscan, then '' in Classical Etr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
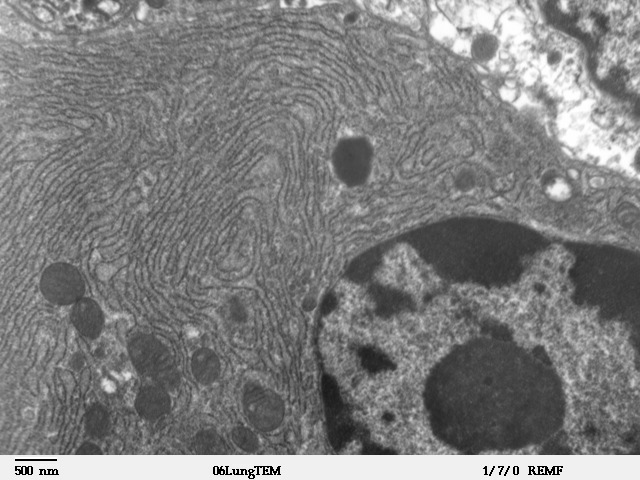
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrion
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, '' Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Envelope
The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material. The nuclear envelope consists of two lipid bilayer membranes: an inner nuclear membrane and an outer nuclear membrane. The space between the membranes is called the perinuclear space. It is usually about 10–50 nm wide. The outer nuclear membrane is continuous with the endoplasmic reticulum membrane. The nuclear envelope has many nuclear pores that allow materials to move between the cytosol and the nucleus. Intermediate filament proteins called lamins form a structure called the nuclear lamina on the inner aspect of the inner nuclear membrane and give structural support to the nucleus. Structure The nuclear envelope is made up of two lipid bilayer membranes, an inner nuclear membrane and an outer nuclear membrane. These membranes are connected to each other by nuclear pores. Two sets of in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Globular Protein
In biochemistry, globular proteins or spheroproteins are spherical ("globe-like") proteins and are one of the common protein types (the others being fibrous, disordered and membrane proteins). Globular proteins are somewhat water-soluble (forming colloids in water), unlike the fibrous or membrane proteins. There are multiple fold classes of globular proteins, since there are many different architectures that can fold into a roughly spherical shape. The term globin can refer more specifically to proteins including the globin fold. Globular structure and solubility The term globular protein is quite old (dating probably from the 19th century) and is now somewhat archaic given the hundreds of thousands of proteins and more elegant and descriptive structural motif vocabulary. The globular nature of these proteins can be determined without the means of modern techniques, but only by using ultracentrifuges or dynamic light scattering techniques. The spherical structure is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined version was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecular Structure
Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length scales ranging from the level of individual atoms to the relationships among entire protein subunits. This useful distinction among scales is often expressed as a decomposition of molecular structure into four levels: primary, secondary, tertiary, and quaternary. The scaffold for this multiscale organization of the molecule arises at the secondary level, where the fundamental structural elements are the molecule's various hydrogen bonds. This leads to several recognizable ''domains'' of protein structure and nucleic acid structure, including such secondary-structure features as alpha helixes and beta sheets for proteins, and hairpin loops, bulges, and internal loops for nucleic acids. The terms ''primary'', ''secondary'', ''tertiary'', and ''q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |