|
C17orf75
Chromosome 17 open reading frame 75 is a protein that in humans is encoded by the ''C17orf75'' gene. ''C17orf75'' is also known as SRI2 (sensitization to ricin complex subunit 2) and is a human protein encoding gene located at 17q11.2 on the complementary strand. The protein this gene encodes is also known as NJMU-R1. The ''C17orf75'' gene is ubiquitously expressed at medium-low levels throughout the body and at slightly higher levels in the brain and testes. This protein is thought to be part of a complex associated with golgin-mediated vesicle capture. Gene The ''C17orf75'' gene spans from position 32,328,441 to position 32,342,173 with a length of 13,733 nucleotides. The length after intron excision is 4,547 nucleotides, and the coding sequence is 1,191 nucleotides in length. ''C17orf75'' has 10 exons. RNA ''C17orf75'' has 4 transcript isoforms: ''C17orf75'' and 3 predicted isoforms which are ''C17orf75'' transcript variant X1 (4,568 nucleotides in length), ''C17orf75'' t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open Reading Frame
In molecular biology, open reading frames (ORFs) are defined as spans of DNA sequence between the start and stop codons. Usually, this is considered within a studied region of a Prokaryote, prokaryotic DNA sequence, where only one of the #Six-frame translation, six possible reading frames will be "open" (the "reading", however, refers to the RNA produced by Transcription (biology), transcription of the DNA and its subsequent interaction with the ribosome in Translation (biology), translation). Such an ORF may contain a start codon (usually AUG in terms of RNA) and by definition cannot extend beyond a stop codon (usually UAA, UAG or UGA in RNA). That start codon (not necessarily the first) indicates where translation may start. The transcription terminator, transcription termination site is located after the ORF, beyond the Translation (biology), translation stop codon. If transcription were to cease before the stop codon, an incomplete protein would be made during translation. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined version was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
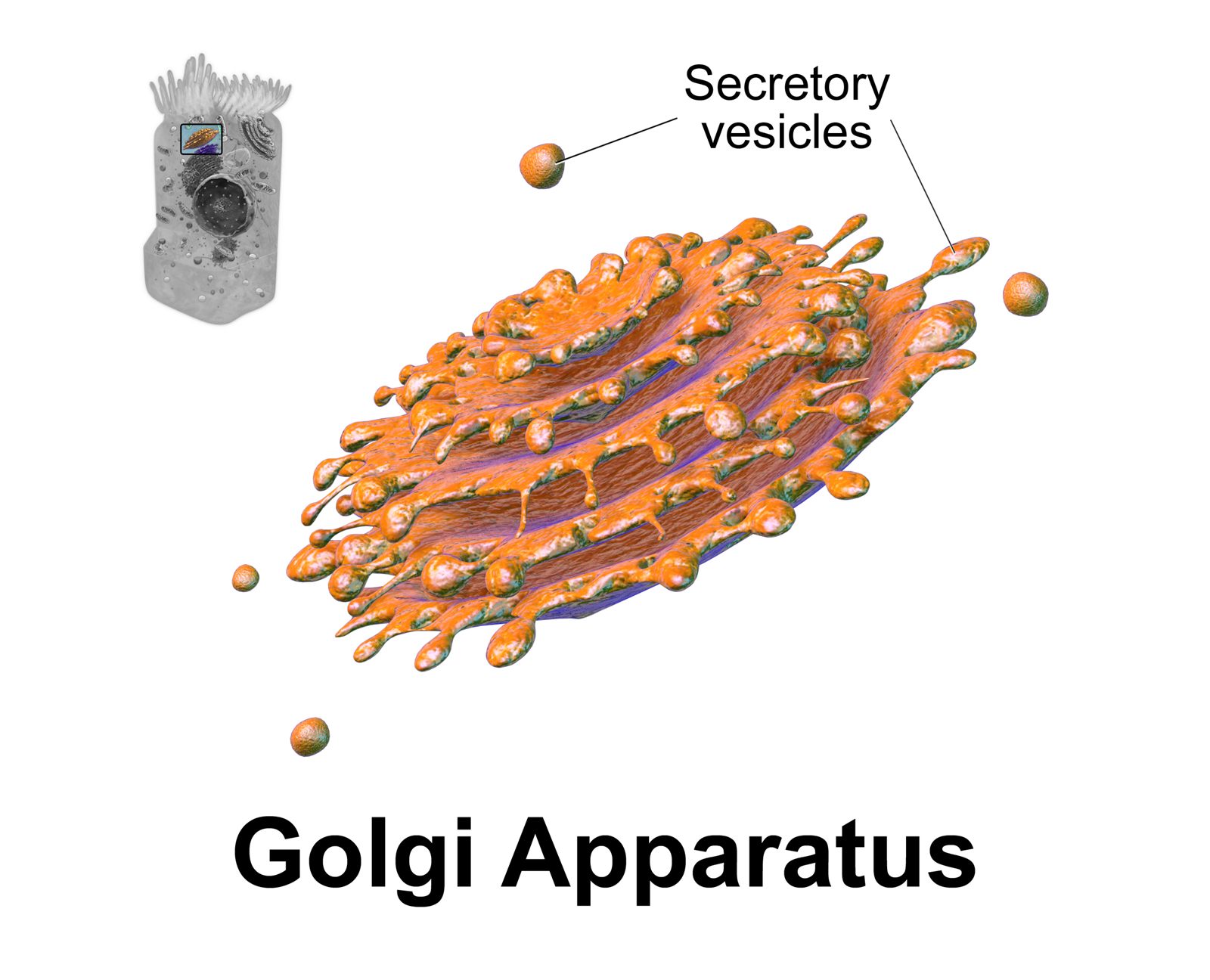
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
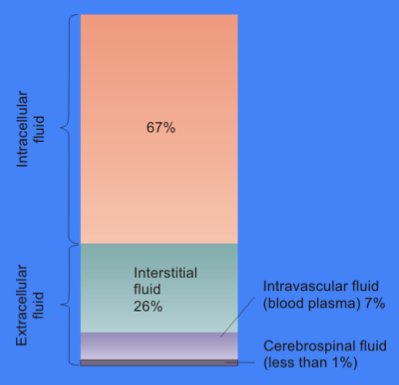
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Circumventricular Organs
Circumventricular organs (CVOs) ( circum-: around ; ventricular: of ventricle) are structures in the brain characterized by their extensive and highly permeable capillaries, unlike those in the rest of the brain where there exists a blood–brain barrier (BBB) at the capillary level. Although the term "circumventricular organs" was originally proposed in 1958 by Austrian anatomist Helmut O. Hofer concerning structures around the brain ventricular system, the penetration of blood-borne dyes into small specific CVO regions was discovered in the early 20th century. The ''permeable'' CVOs enabling rapid neurohumoral exchange include the subfornical organ (SFO), the area postrema (AP), the vascular organ of lamina terminalis (VOLT — also known as the ''organum vasculosum of the lamina terminalis'' (OVLT)), the median eminence, the pituitary neural lobe, and the pineal gland. The circumventricular organs are midline structures around the third and fourth ventricles that a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
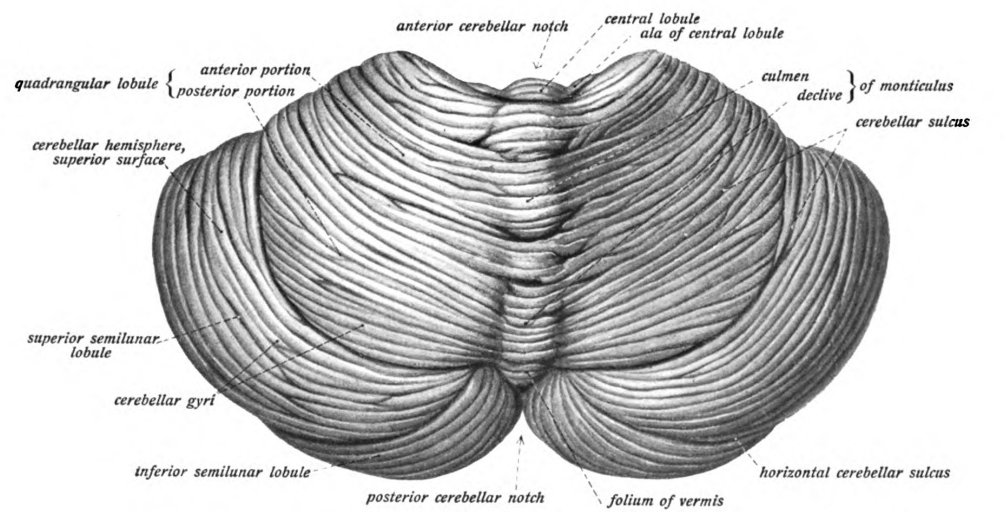
Granular Layer (cerebellum)
The cerebellum (Latin for "little brain") is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as or even larger. In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans. Anatomically, the human cerebellum has the appearance of a separate structure attached to the bottom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purkinje Cell
Purkinje cells, or Purkinje neurons, are a class of GABAergic inhibitory neurons located in the cerebellum. They are named after their discoverer, Czech anatomist Jan Evangelista Purkyně, who characterized the cells in 1839. Structure These cells are some of the largest neurons in the human brain (Betz cells being the largest), with an intricately elaborate dendritic arbor, characterized by a large number of dendritic spines. Purkinje cells are found within the Purkinje layer in the cerebellum. Purkinje cells are aligned like dominos stacked one in front of the other. Their large dendritic arbors form nearly two-dimensional layers through which parallel fibers from the deeper-layers pass. These parallel fibers make relatively weaker excitatory (glutamatergic) synapses to spines in the Purkinje cell dendrite, whereas climbing fibers originating from the inferior olivary nucleus in the medulla provide very powerful excitatory input to the proximal dendrites and cell soma. Pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
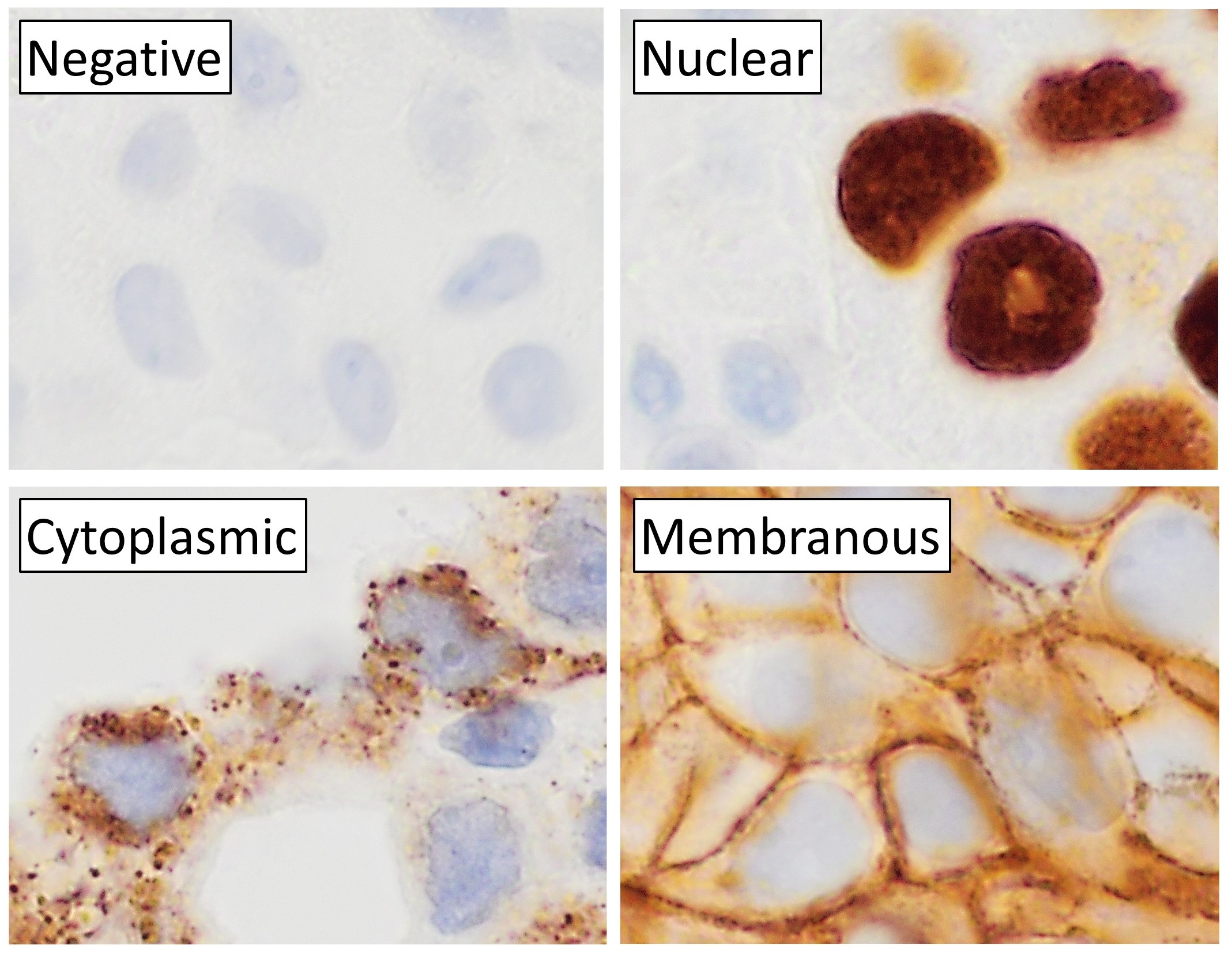
Immunohistochemistry
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue (compare to immunocytochemistry). Albert Coons conceptualized and first implemented the procedure in 1941. Visualising an antibody-antigen interaction can be accomplished in a number of ways, mainly either of the following: * ''Chromogenic immunohistochemistry'' (CIH), wherein an antibody is conjugated to an enzyme, such as peroxidase (the combination being termed immunoperoxidase), that can catalyse a colour-producing reaction. * '' Immunofluorescence'', where the antibody is tagged to a fluorophore, such as fluorescein or rhodamine. Immunohistochemical staining is widely used in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol (), or glycophosphatidylinositol, or GPI in short, is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification. The resulting GPI-anchored proteins play key roles in a wide variety of biological processes. GPI is composed of a phosphatidylinositol group linked through a carbohydrate-containing linker ( glucosamine and mannose glycosidically bound to the inositol residue) and via an ethanolamine phosphate (EtNP) bridge to the C-terminal amino acid of a mature protein. The two fatty acids within the hydrophobic phosphatidyl-inositol group anchor the protein to the cell membrane. Synthesis Glycosylated (GPI-anchored) proteins contain a signal sequence, thus directing them to the endoplasmic reticulum (ER). The protein is co-translationally inserted in the ER membrane via a translocon and is attached to the ER membrane by its hydrophobic C terminus; the majority of the protein extends into the ER ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-linked Glycosylation
''O''-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. ''O''-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzhe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine Sulfation
Tyrosine sulfation is a posttranslational modification where a sulfate group is added to a tyrosine residue of a protein molecule. Secreted proteins and extracellular parts of membrane proteins that pass through the Golgi apparatus may be sulfated. Sulfation was first discovered by Bettelheim in bovine fibrinopeptide B in 1954 and later found to be present in animals and plants but not in prokaryotes or in yeast. Function Sulfation plays a role in strengthening protein-protein interactions. Types of human proteins known to undergo tyrosine sulfation include adhesion molecules, G-protein-coupled receptors, coagulation factors, serine protease inhibitors, extracellular matrix proteins, and hormones. Tyrosine O-sulfate is a stable molecule and is excreted in urine in animals. No enzymatic mechanism of tyrosine sulfate desulfation is known to exist. By knock-out of TPST genes in mice, it may be observed that tyrosine sulfation has effects on the growth of the mice, such as body weight, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)