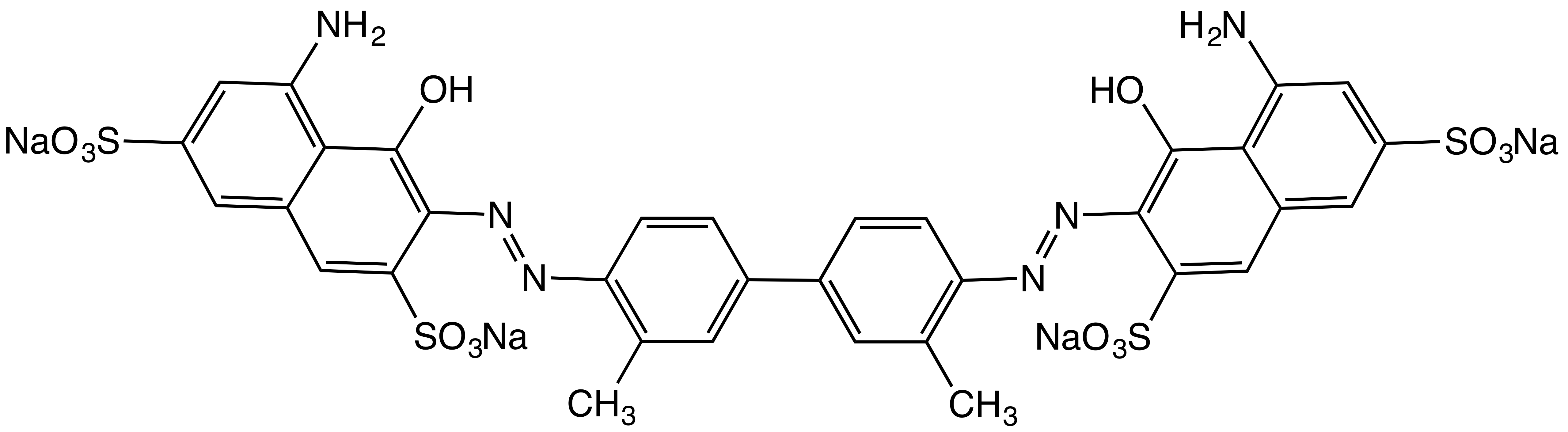
|
Azo Dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food industry, food and textile industry, textile industries. Azo dyes are widely used to treat textile, textiles, leather, leather articles, and some foods. Chemically related derivatives of azo dyes include #Azo pigments, azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-(Phenylazo)phenol Structure
A coxless four, abbreviated as a 4- and also called a straight four, is a racing shell used in the Rowing (sport), sport of competitive rowing. It is designed for four persons who propel the boat with Sweep (rowing), sweep oars, without a coxswain. The crew consists of four rowers, each having one Oar (sport rowing), oar. There are two rowers on the stroke (rowing), stroke side (rower's right hand side) and two on the bow (rowing), bow side (rower's lefthand side). As there is no coxswain, the rudder is controlled by one of the crew, normally with the rudder cable attached to the toe of one of their shoes which can pivot about the ball of the foot, moving the cable left or right. The steersman may row at bow, who has the best vision when looking over their shoulder, or on straighter courses stroke may steer, since they can point the stern of the boat at some landmark at the start of the course. The equivalent boat when it is steered by a coxswain is called a "coxed four". Racin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid-base Indicators
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C ( standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring organic compounds are weak electrolytes, such as carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azoxy
In chemistry, azoxy compounds are a group of organic compounds sharing a common functional group with the general structure . They are considered Amine oxide, N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles and 1,3-dipolar cycloaddition, cycloadd to double bonds. Most azoxy-containing compounds have aryl substituents. Preparation Azoxybenzene and its derivatives are typically prepared by Redox, reduction of Nitro compound, nitrocompounds, such as the reduction of nitrobenzene with arsenous oxide. Such reactions are proposed to proceed via the intermediacy of the nitroso compounds and hydroxylamines, e.g. phenylhydroxylamine and nitrosobenzene (Ph = phenyl, ): PhNHOH + PhNO -> PhN(O)NPh + H2O Nitrosocarbamate esters decarboxylate in strong base to an azotate susceptible to strong alkylation agents: :–N(H)CO2R + 2Nitrogen dioxide, NO2 → –N(N=O)CO2R + HNO3 :–N(N=O)CO2R + KAlkoxide, OR → –N=NO−K+ + CO2 + R2O :–N=NO−K+ + M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetanilide
Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74. Structure Acetoacetanilide crystallizes as the keto-amide tautomer according to X-ray crystallography. The molecules are linked by intermolecular hydrogen bonds, which allows the benzoyl ketone to rotate out of the plane of the amide. For the general case of ''substituted'' acetoanilides, substituents on the aryl ring affect the balance of intra- vs intermolecular hydrogen bonding. The situation is illustrated by the 2' vs. 3' vs. 4' fluoro-substituted acetoacetanilides. Preparation and reactions Acetoacetanilide is prepared by acetoacetylation of aniline using diketene. Many analogues have been prepar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compound where R is hydrogen, is diazenylium. Structure and general properties Arene derivatives According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group raises the ionization constant ''K'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Substitution Reaction
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds and are common ways of introducing functional groups into benzene rings. Some aliphatic compounds can undergo electrophilic substitution as well. Electrophilic aromatic substitution In electrophilic substitution in aromatic compounds, an atom appended to the aromatic ring, usually hydrogen, is replaced by an electrophile. The most important reactions of this type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation and acylation and alkylating Friedel-Crafts reactions. It further consists of alkylation and acylation. Electrophilic aliphatic substitution In electrophilic substitution in aliphatic compounds, an electrophile displaces a functional group. This reaction is similar to nucleophili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activating group, activated carbon (usually from an arene, which is called coupling agent), serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to their extended Conjugated system, conjugated systems. Many are useful dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. In this case, the diazonium ion is degraded by light, leaving a latent image in undegrade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of chemicals, and separation of substances. Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (human-made) resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion exchangers are ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |