|
Azoxy
In chemistry, azoxy compounds are a group of organic compounds sharing a common functional group with the general structure . They are considered Amine oxide, N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles and 1,3-dipolar cycloaddition, cycloadd to double bonds. Most azoxy-containing compounds have aryl substituents. Preparation Azoxybenzene and its derivatives are typically prepared by Redox, reduction of Nitro compound, nitrocompounds, such as the reduction of nitrobenzene with arsenous oxide. Such reactions are proposed to proceed via the intermediacy of the nitroso compounds and hydroxylamines, e.g. phenylhydroxylamine and nitrosobenzene (Ph = phenyl, ): PhNHOH + PhNO -> PhN(O)NPh + H2O Nitrosocarbamate esters decarboxylate in strong base to an azotate susceptible to strong alkylation agents: :–N(H)CO2R + 2Nitrogen dioxide, NO2 → –N(N=O)CO2R + HNO3 :–N(N=O)CO2R + KAlkoxide, OR → –N=NO−K+ + CO2 + R2O :–N=NO−K+ + M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
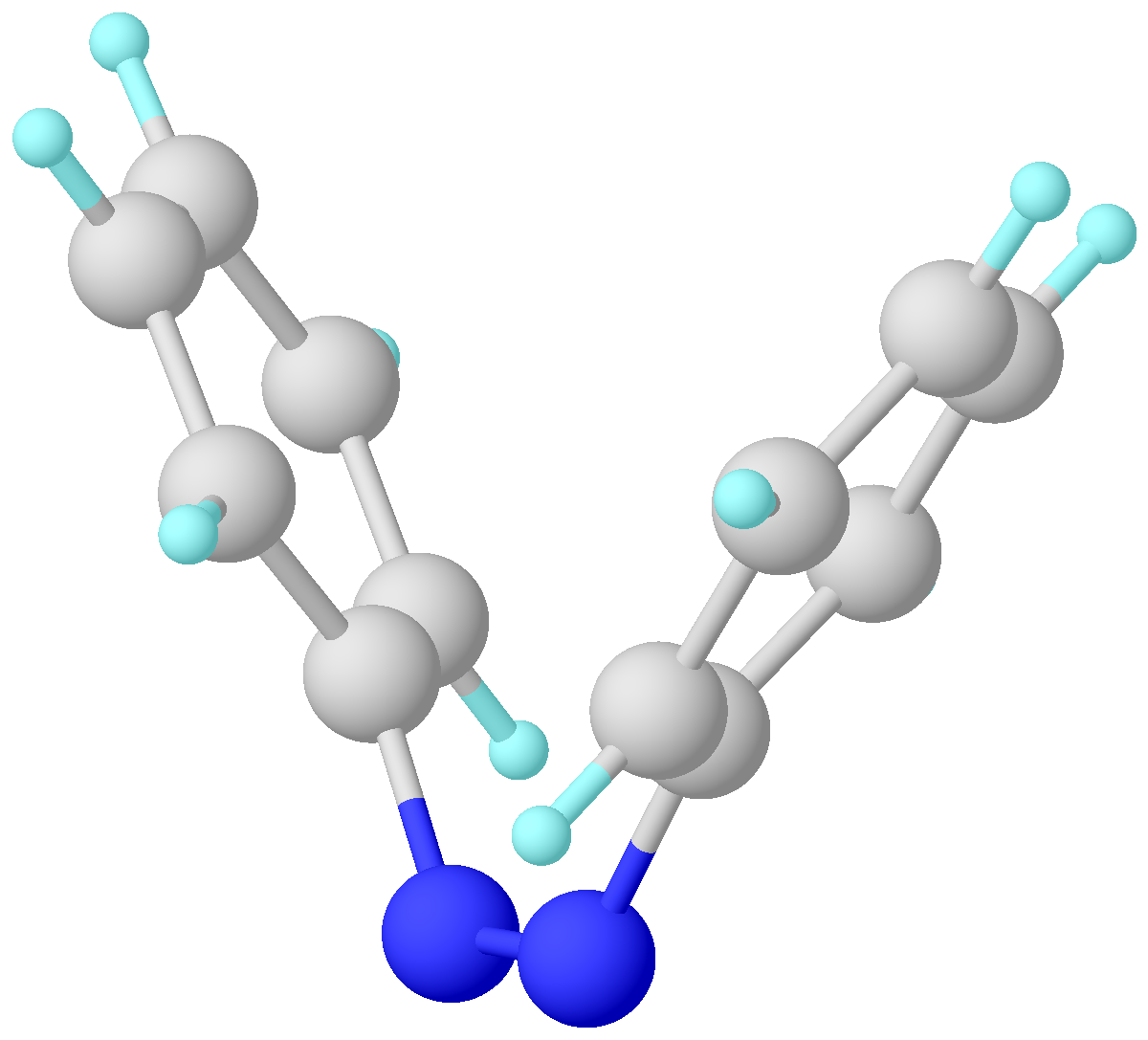
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar Chemical compound, compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings. Structure and synthesis Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. Industrial electrosynthesis using nitrobenzene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine Oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that has the chemical formula . It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-groups attached to nitrogen. Sometimes it is written as or, alternatively, as . In the strict sense, the term ''amine oxide'' applies only to oxides of tertiary amines. Sometimes it is also used for the analogous derivatives of primary and secondary amines. Examples of amine oxides include pyridine-''N''-oxide, a water-soluble crystalline solid with melting point 62–67 °C, and ''N''-methylmorpholine ''N''-oxide, which is an oxidant. Applications Amine oxides are surfactants commonly used in consumer products such as shampoos, conditioners, detergents, and hard surface cleaners. Alkyl dimethyl amine oxide (chain lengths C10–C16) is the most commercially used amine oxide. They are considered a high production volume class o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions. The dipole has at least one resonance structure with positive and negative charges having a 1,3 relationship which can generally be denoted as , where a may be a carbon, oxygen or nitrogen, b may be nitrogen or oxygen, and c may be a carbon, oxygen or nitrogen. Known 1,3-dipoles are: * Azides () * Ozone () * Nitro compounds () * Diazo compounds () * Some oxides ** Azoxide compounds (RN(O)NR) ** Carbonyl oxides ( Criegee zwitterions)Li, Jie Jack''Criegee mechanism of ozonolysis''Book: Name Reactions. 2006, 173-174, ** Nitrile oxides () ** Nitrous oxide () ** Nitrones () * Some imines: ** Azomethine imine ** Nitrilimines (, analogous to nitrile oxide) ** Carbonyl imines * Some ylide An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. As confirmed by X-ray crystallography, nitrobenzene is a planar molecule. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ''H'' = −117 kJ/mol). World capacity for nitrobenzene in 1985 was about 1,700,000 tonnes. The nitration process involves formation of the nitronium ion (NO2+), followed by an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans Isomer
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of". Used alone, trans may refer to: Sociology * Trans, a sociological term which may refer to: ** Transgender, people who identify themselves with a gender that differs from their sex societally designated/assigned at birth ** Transsexual, people who seek to transition from their birth-assigned sex to another via therapy and/or surgery ** Trans*, a broader term for identities including transgender and transsexual Arts, entertainment, and media * Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom * Trans (1982 film), a Venezuelan short documentary film * ''Trans'' (1998 film), an American film * Trans Corp, an Indonesian business unit of CT Corp in the fields of media, lifestyle, and entertainment ** Trans Media, a media subsidiary of Trans Corp *** Trans TV, an Indonesian television network *** Trans7, an Indonesian television network Literature * '' Trans: G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meerwein's Reagent
Triethyloxonium tetrafluoroborate is the organic oxonium compound with the formula . It is often called Meerwein's reagent or Meerwein's salt after its discoverer Hans Meerwein. Also well known and commercially available is the related trimethyloxonium tetrafluoroborate. The compounds are white solids that dissolve in polar organic solvents. They are strong alkylating agents. Aside from the salt, many related derivatives are available. Synthesis and reactivity Triethyloxonium tetrafluoroborate is prepared from boron trifluoride, diethyl ether, and epichlorohydrin: : where the Et stands for ethyl. The trimethyloxonium salt is available from dimethyl ether via an analogous route. These salts do not have long shelf-lives at room temperature. They degrade by hydrolysis: : The propensity of trialkyloxonium salts for alkyl-exchange can be advantageous. For example, trimethyloxonium tetrafluoroborate, which reacts sluggishly due to its low solubility in most compatible solvents, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxy Acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers. Inorganic peroxy acids Peroxymonosulfuric acid (Caro's acid) is probably the most important inorganic peracid, at least in terms of its production scale. It is used for the bleaching of pulp and for the detoxification of cyanide in the mining industry. It is produced by treating sulfuric acid with hydrogen peroxide. Peroxymonophosphoric acid () is prepared similarly. Some peroxy acids are only hypothetical, but their anions are known. This is the case for peroxycarbonate and perborate (see sodium perborate). Organic peracids Production Several organic peroxyacids are commercially useful. They can be prepared in several ways. Most commonly, peracids are ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSD CIF TIHTEK
CSD may refer to: Finance * Central securities depository * Confederate States Dollar * Serbian dinar, by previous ISO 4217 code Organizations Education * California School for the Deaf (other), several institutions * Canyons School District, in Utah, US * Cheltenham School District, in Pennsylvania, US * Christina School District, in Delaware, US * Cleveland School District, in Mississippi, US * Cordova School District, in Alaska, US Other organizations * Canteen Stores Department (India), a chain of stores operated by the Indian Ministry of Defence at military bases * CSD Pakistan (Canteen Stores Department), a chain of stores operated by the Pakistani Ministry of Defence * Chartered Society of Designers, a British learned society for various kinds of design work * Commission on Sustainable Development (1992–2013), a former UN agency * Communication Service for the Deaf, an American non-profit company providing ASL services * Congress of Democratic Trade Un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers. Nitrogen dioxide is poisonous and can be fatal if inhaled in large quantities. Cooking with a gas stove produces nitrogen dioxide which causes poorer indoor air quality. Combustion of gas can lead to increased concentrations of nitrogen dioxide throughout the home environment which is linked to respiratory issues and diseases. The LC50 ( median lethal dose) for humans has been estimated to be 174 ppm for a 1-hour exposure. It is also included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above and becomes a yellowish-brown liquid below . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |