|
Alkene Derivatives
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, distinguished by the position and conformation of the double bond. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical property, chemical or physical property, physical properties. Two main forms of isomerism are structural isomerism, structural (or constitutional) isomerism, in which ''chemical bond, bonds'' between the atoms differ; and stereoisomerism (or spatial isomerism), in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different Isotopologue, isotopologues. The depth of analy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Butene
1-Butene (IUPAC name: But-1-ene, also known as 1-butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas, but easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin (terminal alkene). It is one of the isomers of butene (butylene). It is a precursor to diverse products. Reactions Polymerization of 1-butene gives polybutylene, which is used to make piping for domestic plumbing. Another application is as a comonomer in the production of certain kinds of polyethylene, such as linear low-density polyethylene (LLDPE). It has also been used as a precursor to polypropylene resins, 1,2-Epoxybutane, butylene oxide, and butanone. Manufacturing 1-Butene is produced by separation from crude C4 refinery streams and by ethylene dimerization. The former affords a mixture of 1-and 2-butenes, while the latter affords only the terminal alkene. It is distilled to give a very high purity product. An estimated 12 billion kilograms were pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor. Propylene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust. It was discovered in 1850 by A. W. von Hoffmann's student Captain (later Major General) John Williams Reynolds as the only gaseous product of thermal decomposition of amyl alcohol to react with chlorine and bromine. Production Steam cracking The dominant technology for producing propylene is steam cracking, using propane as the feedstock. Cracking propane yields a mixture of ethylene, propylene, methane, hydrogen gas, and other related compounds. The yield of propylene is about 15%. The other principal feedstock is naphtha, especially in the Middle East and Asia. Propylene can be separated by fractional distill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the ring (chemistry), monocyclic Saturated and unsaturated compounds, saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single bond, single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by IUPAC, the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014)''Dictionary of the History of Science'' page 218. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
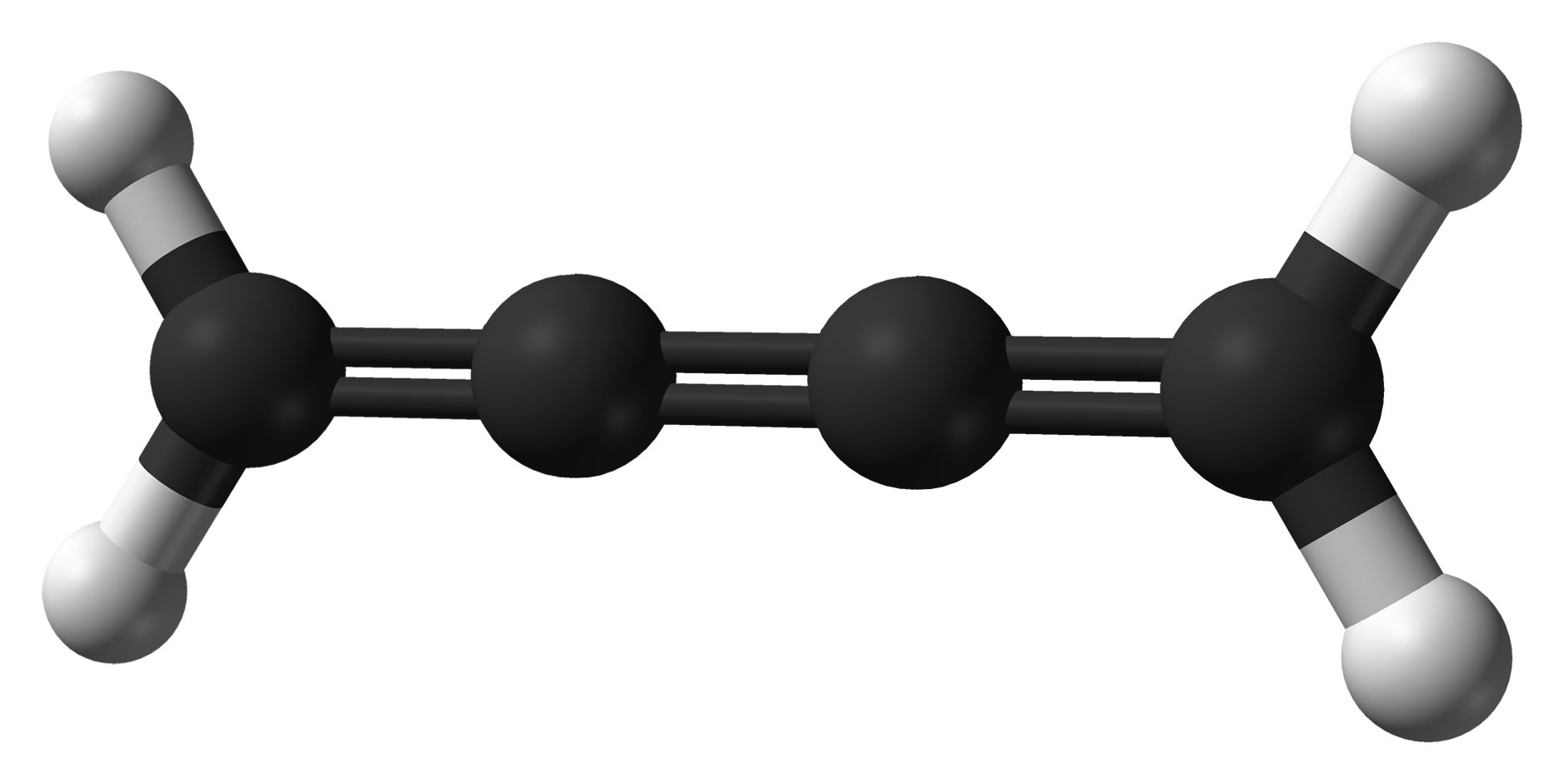
Cumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular cloud A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...s and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reporte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propadiene
Propadiene () or allene () is the organic compound with the formula . It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding. Production and equilibrium with methylacetylene Propadiene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for methylacetylene-propadiene: : for which at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, of dehydrogenation of propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ... polymerization of propene. Occurrence in Space In 2019 it wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allenes
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is H or some organyl group). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene (), which is itself also called ''allene''. A group of the structure is called allenyl, while a substituent attached to an allene is referred to as an allenic substituent (R is H or some alkyl group). In analogy to allylic and propargylic, a substituent attached to a saturated carbon α (i.e., directly adjacent) to an allene is referred to as an allenylic substituent. While allenes have two consecutive ('cumulated') double bonds, compounds with three or more cumulated double bonds are called cumulenes. History For many years, allenes were viewed as curiosities but thought to be synthetically useless and difficult to prepare and to work with.The Chemistry of the Allenes (vol. 1−3); Landor, S. R., Ed.; cademi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
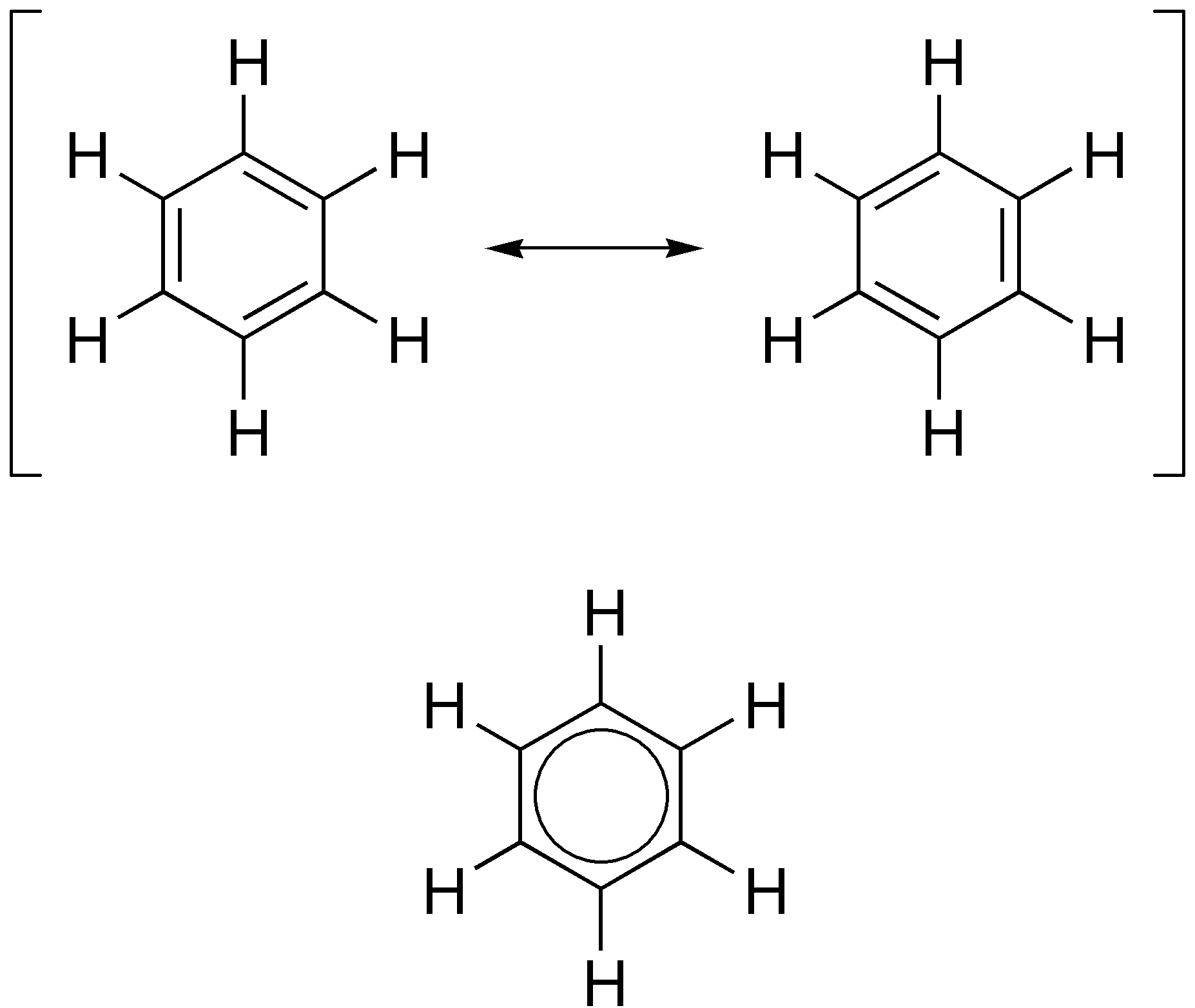
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations. Preferred IUPAC names are applicable only for organic compounds, to which the IUPAC (International Union of Pure and Applied Chemistry) has the definition as compounds which contain at least a single carbon atom but no alkali, alkaline earth or transition metals and can be named by the nomenclature of organic compounds (see below). Rules for the remaining organic and inorganic compounds are still under development. The concept of PINs is defined in the introductory chapter and chapter 5 of the ''"Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013"'' (fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |