|
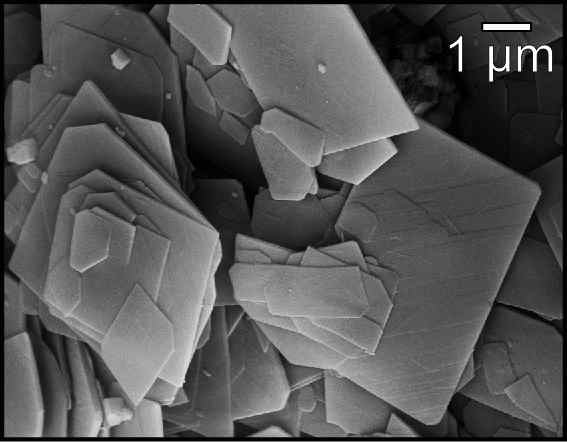
Brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Magnesium, Mg(hydroxyl, OH)2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal Vein (geology), vein mineral in metamorphosed limestones and Chlorite group, chlorite schists; and formed during serpentinization of dunites. Brucite is often found in association with Serpentine group, serpentine, calcite, aragonite, Dolomite (mineral), dolomite, magnesite, hydromagnesite, artinite, talc and chrysotile. It adopts a layered CdI2-like structure with hydrogen-bonds between the layers. Discovery Brucite was first described in 1824 by François Sulpice Beudant and named for the discoverer, American mineralogist, Archibald Bruce (mineralogist), Archibald Bruce (1777–1818). A fibrous variety of brucite is called nemalite. It occurs in fibers or laths, usually elongated along [1010], but sometimes [1120] Miller index, crystalline directions. Occurrence A notable location i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serpentinization
Serpentinization is a hydration and Metamorphic rock, metamorphic transformation of ferromagnesian minerals, such as olivine and pyroxene, in mafic and ultramafic rock to produce serpentinite. Minerals formed by serpentinization include the Serpentine subgroup, serpentine group minerals (antigorite, lizardite, chrysotile), brucite, talc, Ni-Fe alloys, and magnetite. The mineral alteration is particularly important at the sea floor at plate tectonics, tectonic plate boundaries. Formation and petrology Serpentinization is a form of low-temperature (0 to ~600 °C) metamorphism of ferromagnesian minerals in mafic and ultramafic rocks, such as dunite, harzburgite, or lherzolite. These are rocks low in silica and composed mostly of olivine (), pyroxene (), and chromite (approximately ). Serpentinization is driven largely by Mineral hydration, hydration and oxidation of olivine and pyroxene to serpentine subgroup, serpentine group minerals (antigorite, lizardite, and chrysotile), bru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the Precipitation (chemistry), precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2 OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with calcium hydroxide, lime (Ca(OH)2). A volume of of seawater gives about of Mg(OH)2. Ca(OH)2 ) is far more soluble than Mg(OH)2 ) and dramatically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorite Group
The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in Mineral alteration, altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite. Chlorite minerals show a wide variety of compositions, in which magnesium, iron, aluminium, and silicon substitute for each other in the crystal structure. A complete solid solution series exists between the two most common end members, magnesium-rich clinochlore and iron-rich chamosite. In addition, manganese, zinc, lithium, and calcium species are known. The great range in composition results in considerable variation in physical, optical, and X-ray diffraction, X-ray properties. Similarly, the range of chemical composition allows chlorite group minerals to exist over a wide range of temperature and pressure conditions. For this reason chlorite minerals are ubiquitous minerals within low and medium temperat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talc
Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula . Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant. It is an ingredient in ceramics, paints, and roofing material. It is a main ingredient in many cosmetics. It occurs as foliated to fibrous masses, and in an exceptionally rare crystal form. It has a perfect basal cleavage and an uneven flat fracture, and it is foliated with a two-dimensional platy form. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 1 as the hardness of talc, the softest mineral. When scraped on a streak plate, talc produces a white streak, though this indicator is of little importance, because most silicate minerals produce a white streak. Talc is translucent to opaque, with colors ranging from whitish grey to green with a vitreous and pearly luster. Talc is not soluble i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydromagnesite
Hydromagnesite is a hydrated magnesium carbonate mineral with the formula . It generally occurs associated with the weathering products of magnesium containing minerals such as serpentine group, serpentine or brucite. It occurs as incrustations and vein or fracture fillings in ultramafic rocks and serpentinites, and occurs in hydrothermally altered Dolomite (rock), dolomite and marble. Hydromagnesite commonly appears in caves as speleothems and "moonmilk", deposited from water that has seeped through magnesium rich rocks. It is the most common cave carbonate after calcite and aragonite. The mineral thermally decomposes, over a temperature range of approximately 220 °C to 550 °C, releasing water and carbon dioxide leaving a magnesium oxide residue. Hydromagnesite was first described in 1836 for an occurrence in Hoboken, New Jersey. Stromatolites in an alkaline (pH greater than 9) freshwater lake (Lake Salda, Salda Gölü) in southern Turkey are made of hydromagnesite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artinite
Artinite is a hydrated basic magnesium carbonate mineral with formula: . It forms white silky monoclinic prismatic crystals that are often in radial arrays or encrustations. It has a Mohs hardness of 2.5 and a specific gravity of 2. It occurs in low-temperature hydrothermal veins and in serpentinite, serpentinized ultramafic rocks. Associated minerals include brucite, hydromagnesite, pyroaurite, chrysotile, aragonite, calcite, Dolomite (mineral), dolomite and magnesite. It was first reported in 1902 in Lombardy, Italy. It was named for Italian mineralogist, Ettore Artini (1866–1928). References Magnesium minerals Carbonate minerals Hydroxide minerals Trihydrate minerals Monoclinic minerals Minerals in space group 12 Minerals described in 1902 {{carbonate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periclase
Periclase is a magnesium mineral that occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide ( Mg O). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite. It was first described in 1840 and named from the Greek περικλάω (to break around) in allusion to its cleavage. The type locality is Monte Somma, Somma-Vesuvius Complex, Naples Province, Campania, Italy. The old term for the mineral is ''magnesia''. Stones from the Magnesia region in ancient Anatolia contained both magnesium oxide and hydrated magnesium carbonate as well as iron oxides (such as magnetite). Thus these stones, called ''Stones from Magnesia'' in antiquity, with their unusual magnetic properties were the reason the terms ''magnet'' and ''magnetism'' were coined. Periclase is usually found in marble produced by metamorphism of dolomitic lim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The Geology, geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock (geology), rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate (geology), aggregate of two or more different types of minerals, spaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
François Sulpice Beudant
François Sulpice Beudant (5 September 1787 – 10 December 1850) was a French mineralogist and geologist. The mineral beudantite was named after him. Life Born in Paris, he was educated at the Ecole Polytechnique and Ecole Normale, and in 1811 was appointed professor of mathematics at the lycée of Avignon. Thence he was called, in 1813, to the lycée of Marseille to fill the post of professor of physics, where he carried out the first measurements of the speed of sound in seawater. In the following year the royal mineralogical cabinet was committed to his charge to be conveyed into England, and from that time his attention was directed principally towards geology and cognate sciences. In 1817, he published a paper on the phenomena of crystallization, treating especially of the variety of forms assumed by the same mineral substance. In 1818 he undertook, at the expense of the French government, a geological journey through Hungary, and the results of his researches, ''Voyage mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
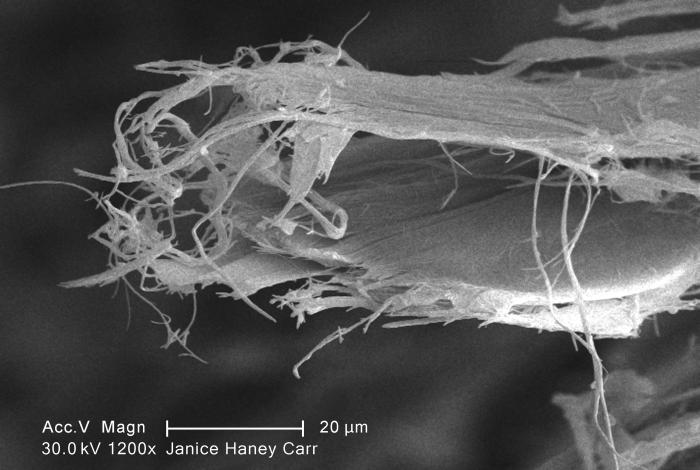
Chrysotile
Chrysotile or white asbestos is the most commonly encountered form of asbestos, accounting for approximately 95% of the asbestos in the United StatesOccupational Safety and Health Administration, U.S. Department of Labor (2007)29 C.F.R. 1910.1001 Appendix J. and a similar proportion in other countries.Institut national de recherche sur la sécurité (1997).Amiante." ''Fiches toxicologiques.'' n° 167. (in French) It is a soft, fibrous silicate mineral in the serpentine subgroup of phyllosilicates; as such, it is distinct from other asbestiform minerals in the amphibole group. Its idealized chemical formula is Mg( Si O)( OH). The material has physical properties which make it desirable for inclusion in building materials, but poses serious health risks when dispersed into air and inhaled. Polytypes Three polytypes of chrysotile are known. These are very difficult to distinguish in hand specimens, and polarized light microscopy must normally be used. Some older publ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |