|
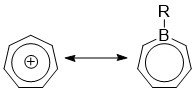
Borepin
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene. This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid. The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
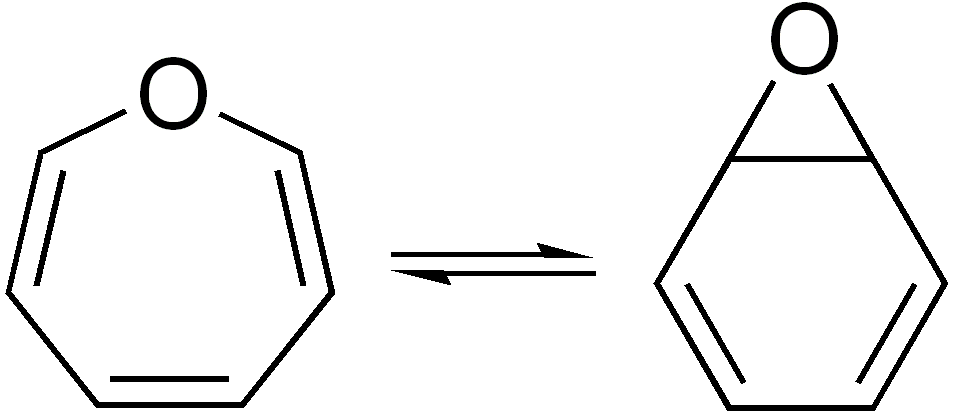
Borepin Valence Isomer
Borepins are a class of boron-containing Heterocyclic compound, heterocycles used in Main-group element, main group chemistry. They consist of a seven-membered Saturated and unsaturated compounds, unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of Pi bond, 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the Molecular orbital, π and π* orbitals of the cycloheptatriene. This leads to an Isoelectronicity, isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acids and bases, Lewis acid. The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropylium Cation
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (''see below''). Its bromide and chloride salts can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively. It is a regular heptagonal, planar, cyclic ion. It has 6 π-electrons (4''n'' + 2, where ''n'' = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms. The structure shown is a composite of seven resonance contributors in which each carbon atom carries part of the positive charge. History In 1891 G. Merling obtained a water-soluble bromine-containing compound from the reaction of cycloheptatriene and bromine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–750 nm) or infrared radiation (750–2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics. Concept Grotthuss–Draper law and Stark-Einstein law Photoexcitation is the first step in a photochemical process where the reactant is elevat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pericyclic Reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap in a continuous cycle at the transition state. Pericyclic reactions stand in contrast to ''linear reactions'', encompassing most organic transformations and proceeding through an acyclic transition state, on the one hand and '' coarctate reactions'', which proceed through a doubly cyclic, concerted transition state on the other hand. Pericyclic reactions are usually rearrangement or addition reactions. The major classes of pericyclic reactions are given in the table below (the three most important classes are shown in bold). Ene reactions and cheletropic reactions are often classed as group transfer reactions and cycloadditions/cycloeliminations, respectively, while dyotropic reactions and group transfer reactions (if ene reactions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions. Benzene There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions. Image:Benzene-2D-flat.png, Benzene Image:Historic Benzene Formulae Dewar(1867) V.1.svg, Dewar benzene Image:Prisman2.svg, Prismane Image:Benzvalene.png, Benzvalene Image:Bicycloprop-2-enyl.svg, Bicyclopropenyl Cyclooctatetraene The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Image:Cyclooct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holger Braunschweig
Holger Braunschweig is Head and Chair of Inorganic Chemistry at the Julius-Maximilians-University of Würzburg in Würzburg, Germany. He is best known for founding the field of transition metal-boron multiple bonding (transition metal borylenes), the synthesis of the first stable compounds containing boron-boron and boron-oxygen triple bonds, the isolation of the first non-carbon/nitrogen main-group dicarbonyl, and the first fixation of dinitrogen at an element of the p-block of the periodic table. By modifying a strategy pioneered by Prof. Gregory Robinson of the University of Georgia, Braunschweig also discovered the first rational and high-yield synthesis of neutral compounds containing boron-boron double bonds (diborenes). In 2016 Braunschweig isolated the first compounds of beryllium in the oxidation state of zero. Education and research career Braunschweig obtained his Ph.D. and Habilitation from RWTH Aachen with P. Paetzold and worked as a postdoctoral researche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring Current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field. The ring current creates its own magnetic field. Outside the ring, this field is in the same direction as the externally applied magnetic field; inside the ring, the field counteracts the externally applied field. As a result, the net magnetic field outside the ring is greater than the externally applied field alone, and is less inside the ring. Aromatic ring currents are relevant to NMR spectroscopy, as they dramatically influence the chemical shifts of 1H nuclei ("protons") ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present ( heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minimal Sub
{{disambiguation ...
Minimal may refer to: * Minimal (music genre), art music that employs limited or minimal musical materials * "Minimal" (song), 2006 song by Pet Shop Boys * Minimal (supermarket) or miniMAL, a former supermarket chain in Germany and Poland * Minimal (''Dungeons & Dragons''), a creature of magically reduced size in the game ''Dungeons & Dragons'' * Minimal (chocolate), a bean to bar chocolate store in Japan, featured in '' Kantaro: The Sweet Tooth Salaryman'' * Minimal (clothing), an Indonesia clothing-retail company that worked with fashion model Ayu Gani See also * *Minimalism (other) *Maximal (other) *Minimisation (other) *Minimal prime (other) In mathematics, the term minimal prime may refer to *Minimal prime ideal, in commutative algebra *Minimal prime (recreational mathematics) In recreational number theory, a minimal prime is a prime number for which there is no shorter subsequence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
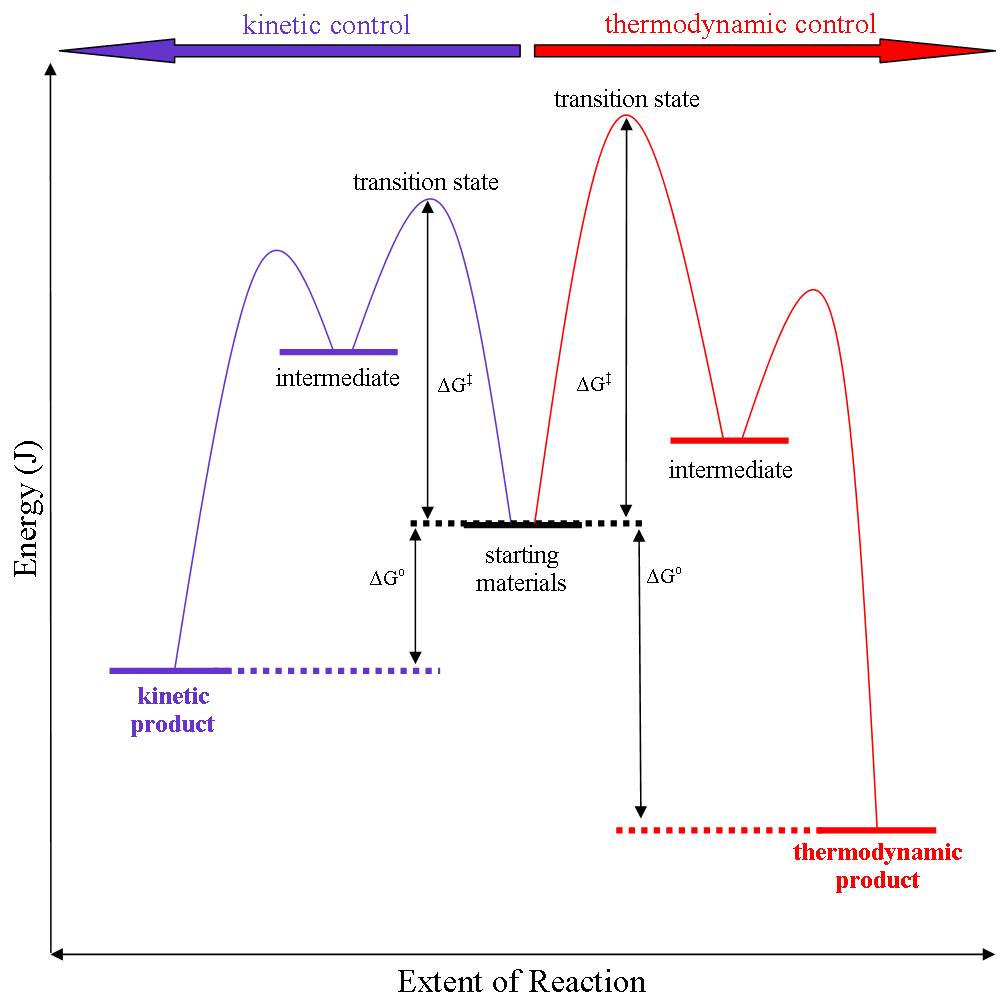
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conrotatory And Disrotatory
An electrocyclic reaction can either be classified as conrotatory or disrotatory based on the rotation at each end of the molecule. In conrotatory mode, both atomic orbitals of the end groups turn in the same direction (such as both atomic orbitals rotating clockwise or counter-clockwise). In disrotatory mode, the atomic orbitals of the end groups turn in opposite directions (one atomic orbital turns clockwise and the other counter-clockwise). The cis/trans geometry of the final product is directly decided by the difference between conrotation and disrotation. Determining whether a particular reaction is conrotatory or disrotatory can be accomplished by examining the molecular orbitals of each molecule and through a set of rules. Only two pieces of information are required to determine conrotation or disrotation using the set of rules: how many electrons are in the pi-system and whether the reaction is induced by heat or by light. This set of rules can also be derived from an ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |