|
Benzoxazoles
Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine. Although benzoxazole itself is of little practical value, many derivatives of benzoxazoles are commercially important. Being a heterocyclic compound, benzoxazole finds use in research as a starting material for the synthesis of larger, usually bioactive structures. Its aromaticity makes it relatively stable, although as a heterocycle, it has reactive sites which allow for functionalization. Occurrence and applications It is found within the chemical structures of pharmaceutical drugs such as flunoxaprofen and tafamidis. Benzoxazole derivatives are also of interest for optical brighteners in laundry detergents.E. Smulders, E. Sung "Laundry Detergents, 2. Ingredients and Products" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Benzoxazoles belong to the group of well-known antifungal agents with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flunoxaprofen
Flunoxaprofen, also known as Priaxim, is a chiral nonsteroidal anti-inflammatory drug (NSAID). It is closely related to naproxen, which is also an NSAID. Flunoxaprofen has been shown to significantly improve the symptoms of osteoarthritis and rheumatoid arthritis. The clinical use of flunoxaprofen has ceased due to concerns of potential hepatotoxicity. Structure Flunoxaprofen is a two-ring heterocyclic compound derived from benzoxazole. It also contains a fluorine atom and a propanoyl group. Synthesis The overall synthesis is similar to that for benoxaprofen; in this case, para-fluorobenzoyl chloride is used when forming the benzoxazole ring.. : A Sandmeyer reaction by diazotisation of 2-(4-aminophenyl)propanenitrile (1) followed by acid hydrolysis leads to the phenol (2), which is nitrated and reduced using stannous chloride or catalytic hydrogenation to give the aminophenol (4). Hydrolysis of the nitrile produces the carboxylic acid (5), which is converted to racemic fluno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tafamidis
Tafamidis, sold under the brand names Vyndaqel and Vyndamax, is a medication used to delay disease progression in adults with certain forms of transthyretin amyloidosis. It can be used to treat both hereditary forms, familial amyloid cardiomyopathy and familial amyloid polyneuropathy, as well as wild-type transthyretin amyloidosis, which formerly was called senile systemic amyloidosis. It works by stabilizing the quaternary structure of the protein transthyretin. In people with transthyretin amyloidosis, transthyretin falls apart and forms clumps called (amyloid) that harm tissues including nerves and the heart. The US Food and Drug Administration considers tafamidis to be a first-in-class medication. Medical use Tafamidis is used to delay nerve damage in adults who have transthyretin amyloidosis with polyneuropathy, or heart disease in adults who have transthyretin amyloidosis with cardiomyopathy. It is taken by mouth. Women should not get pregnant while taking it and sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazole
Oxazole is the parent compound for a vast class of heterocyclic compound, heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa, p''K''a of 0.8, compared to 7 for imidazole. Preparation The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones: The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used: Other methods are known including the reaction of α-haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC. Biosynthesis In biomolecules, oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides: : Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom. Reactions With a pKa of 0.8 for the conjugate acid (oxazolium salts), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Bases
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
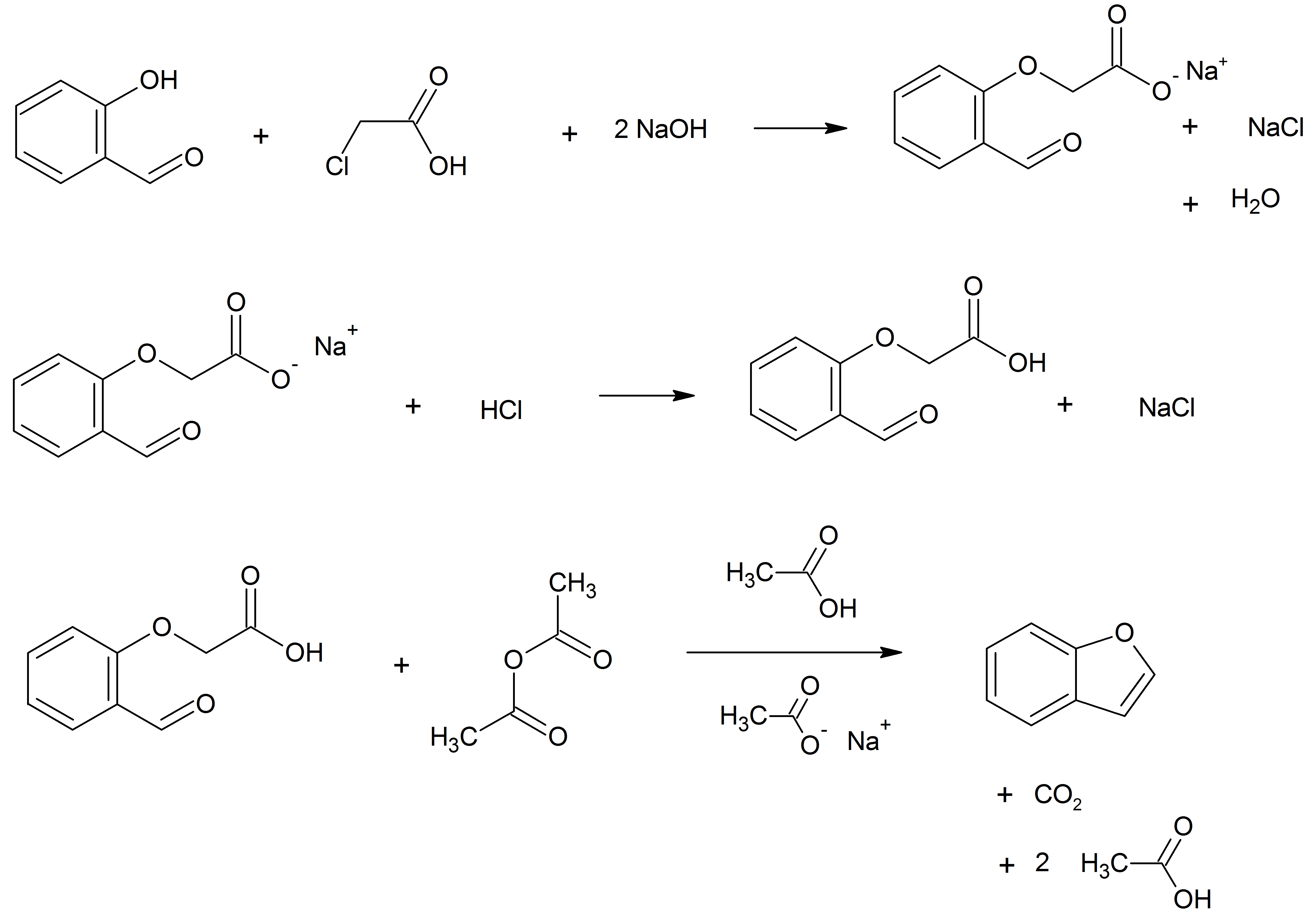
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. It has been identified in cannabis. It is the main volatile compound in stinky tofu. When indole is a substituent on a larger molecule, it is called an ''indolyl group'' by systematic nomenclature. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiazole
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole. It has a sulfurous odor and meaty flavor. The three structural isomers of benzothizaole are 1,3-benzothiazole, 1,2-benzothiazole and 2,1-benzothiazole. Structure and reactivity Benzothiazoles consist of a 5-membered 1,3- thiazole ring fused to a benzene ring. The nine atoms of the bicycle and the attached substituents are coplanar. The heterocyclic core of the molecule is readily substituted at the methyne (CH) centre in the thiazole ring. Thiazole is electron-withdrawing. Synthesis and biosynthesis Benzothiazoles are typically prepared by treatment of 2-mercaptoaniline. For example, acid chlorides are effective: :C6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a white solid that appears in form of tabular crystals. Preparation Benzimidazole was discovered during research on vitamin B12. The benzimidazole nucleus was found to be a stable platform on which drugs could be developed. Benzimidazole is produced by Condensation reaction, condensation of o-phenylenediamine with formic acid, or the equivalent trimethyl orthoformate: :C6H4(NH2)2 + HC(OCH3)3 → C6H4N(NH)CH + 3 CH3OH 2-Substituted derivatives are obtained when the condensation is conducted with aldehydes in place of formic acid, followed by oxidation. Reactions Benzimidazole is a Base (chemistry), base: :C6H4N(NH)CH + H+ → [C6H4(NH)2CH]+ It can also be deprotonated with stronger bases: :C6H4N(NH)CH + LiH → Li [C6H4N2CH] + H2 The imine can be alkylated and also serves as a ligand in coordinati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzisoxazole
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide. Its aromaticity makes it relatively stable; however, it is only weakly basic. Synthesis Benzisoxazole may be prepared from inexpensive salicylaldehyde, via a base catalyzed room temperature reaction with hydroxylamine-''O''-sulfonic acid. : Reactions Kemp elimination First reported by Daniel S. Kemp, the relatively weak N-O bond can be cleaved by a strong base to yield a 2-hydroxybenzonitrile species. See also ;Structural isomers *Benzoxazole *Anthranil Anthranil (2,1-benzisoxazole) is an organic compound with a molecular formula C7H5NO, which fea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |