|
Arsenic Pentafluoride
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5. Synthesis Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...: :2As + 5F2 → 2AsF5 It can also be prepared by the reaction of arsenic trifluoride and fluorine: :AsF3 + F2 → AsF5 or the addition of fluorine to arsenic pentoxide or arsenic trioxide. :2As2O5 + 10F2 → 4AsF5 + 5O2 :2As2O3 + 10F2 → 4AsF5 + 3O2 Properties Arsenic pentafluoride is a colourless gas and has a trigonal bipyramidal structure. In the solid state the axial As−F bond lengths are 171.9 pm and the equatorial 166.8 pm. Its point group is D3h. Reactions Arsenic pentafluoride form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic(V) Compounds
Arsenic is a chemical element; it has symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is notoriously toxic. It occurs naturally in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. It has various allotropes, but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is also a common n-type dopant in semiconductor electronic devices, and a component of the III–V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the persistent toxicity of arsenic and its compounds. Arsen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Highly Toxic Gases
Many gases have toxic properties, which are often assessed using the LC50 (median lethal concentration) measure. In the United States, many of these gases have been assigned an NFPA 704 health rating of 4 (may be fatal) or 3 (may cause serious or permanent injury), and/or exposure limits ( TLV, TWA/PEL, STEL, or REL) determined by the ACGIH professional association. Some, but by no means all, toxic gases are detectable by odor, which can serve as a warning. Among the best known toxic gases are carbon monoxide, chlorine, nitrogen dioxide and phosgene. Definitions *Toxic: a chemical that has a median lethal concentration (LC50) in air of more than 200 parts per million (ppm) but not more than 2,000 parts per million by volume of gas or vapor, or more than 2 milligrams per liter but not more than 20 milligrams per liter of mist, fume or dust, when administered by continuous inhalation for 1 hour (or less if death occurs within 1 hour) to albino rats weighing between 200 and 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroarsenate
The hexafluoroarsenate (sometimes shortened to fluoroarsenate) anion is a chemical species with formula . Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid (). Synthesis The first undisputed synthesis is due to Otto Ruff, Kurt Stäuber and Hugo Graf, who began with the lower-valent arsenic trifluoride, using silver(I) fluoride as both a fluorine source and oxidant: cites but discounts it as describing an implausibly easy synthesis with a hydrolyzable product. : In the following reaction, one mole of arsenic trifluoride, three moles of silver fluoride, and one mole of nitrosyl chloride are reacted to produce one mole of nitrosyl hexafluoroarsenate, one mole of silver chloride, and two moles of elemental silver. Modern syntheses usually begin with arsenic pentafluoride (), which abstracts fluoride from common donors, such as hydrogen fluoride () or ''cis''- difluorodiazine (). Although the hexafluoroarsenate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
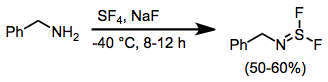
Sulfur Tetrafluoride
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
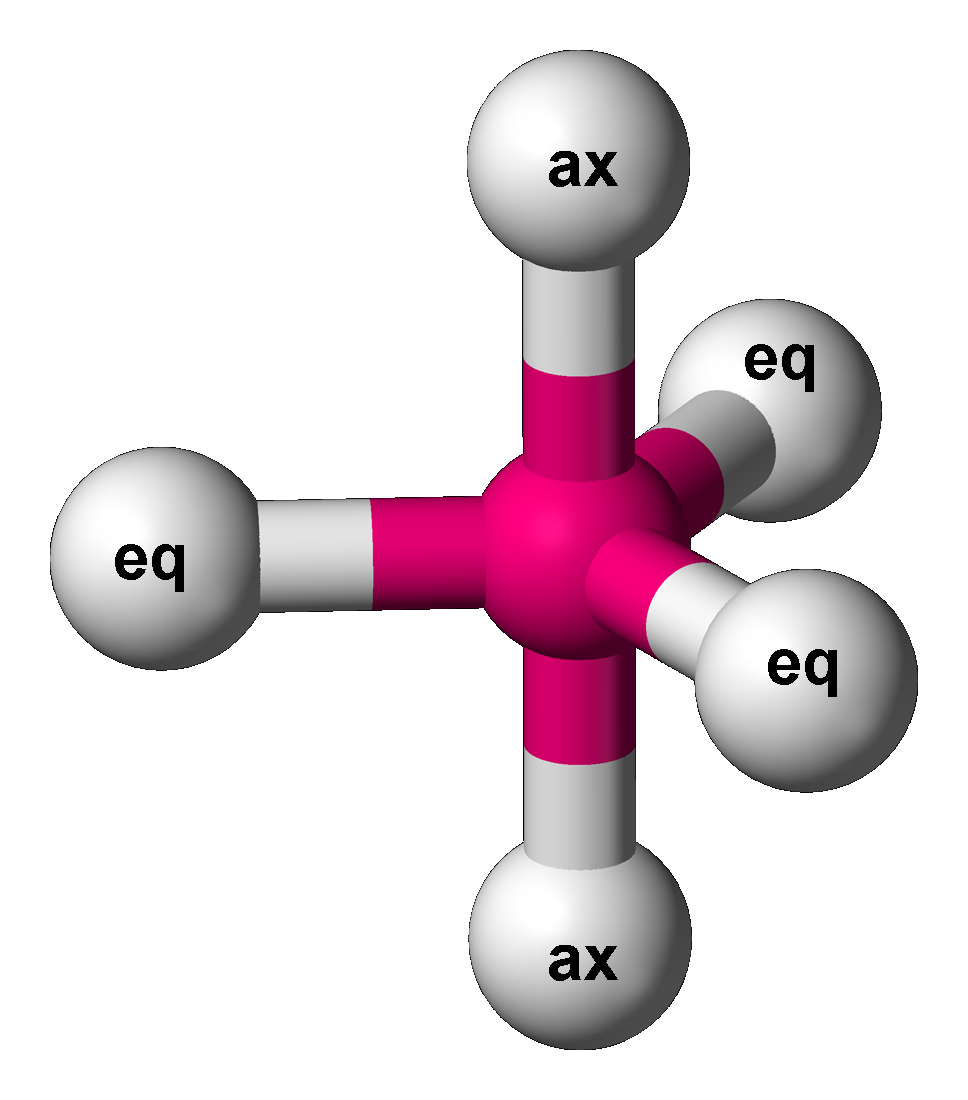
Trigonal Bipyramidal
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Trioxide
Arsenic trioxide is an inorganic compound with the formula . As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. It is sold under the brand name Trisenox among others when used as a medication to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein. Common side effects include vomiting, diarrhea, swelling, shortness of breath, and headaches. Severe side effects may include APL differentiation syndrome and heart problems. Use during pregnancy or breastfeeding may harm the baby. Its mechanism in treating cancer is not entirely clear. Arsenic trioxide was approved for medical use in the United States in 2000. It is on the World Health Organization's List of Essential Medicines. Approximately 50,000 tonnes are produced a year. Due to its toxicity, a number of countries have regulations around its manufacture and sale. Uses Medical Arsenic trioxide is indicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Pentoxide
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications. Structure The structure consists of tetrahedral and octahedral centers linked by sharing corners. The structure differs from that of the corresponding Phosphorus pentoxide, phosphorus(V) oxide; as a result, although there is still a solid solution with that oxide, it only progresses to the equimolar point, at which point phosphorus has substituted for arsenic in all of its tetrahedral sites. Likewise, arsenic pentoxide can also dissolve up to an equimolar amount of antimony pentoxide, as antimony substitutes for arsenic only in its octahedral sites. Synthesis Historical Pierre Macquer found a crystallizable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Trifluoride
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. Like other inorganic arsenic compounds, it is highly toxic. Preparation and properties It can be prepared by reacting hydrogen fluoride, HF, with arsenic trioxide Arsenic trioxide is an inorganic compound with the formula . As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. It is sold under the brand name Trisenox among others when used as a m ...: :6HF + As2O3 → 2AsF3 + 3H2O It has a pyramidal molecular structure in the gas phase which is also present in the solid. In the gas phase the As-F bond length is 170.6 pm and the F-As-F bond angle 96.2°. Arsenic trifluoride is used as a fluorinating agent for the conversion of non-metal chlorides to fluorides, in this respect it is less reactive than SbF3. Salts containing AsF4− anion can be prepared for ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is notoriously toxic. It occurs naturally in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. It has various Allotropes of arsenic, allotropes, but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is also a common n-type dopant in semiconductor electronic devices, and a component of the III–V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the persistent tox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |