|
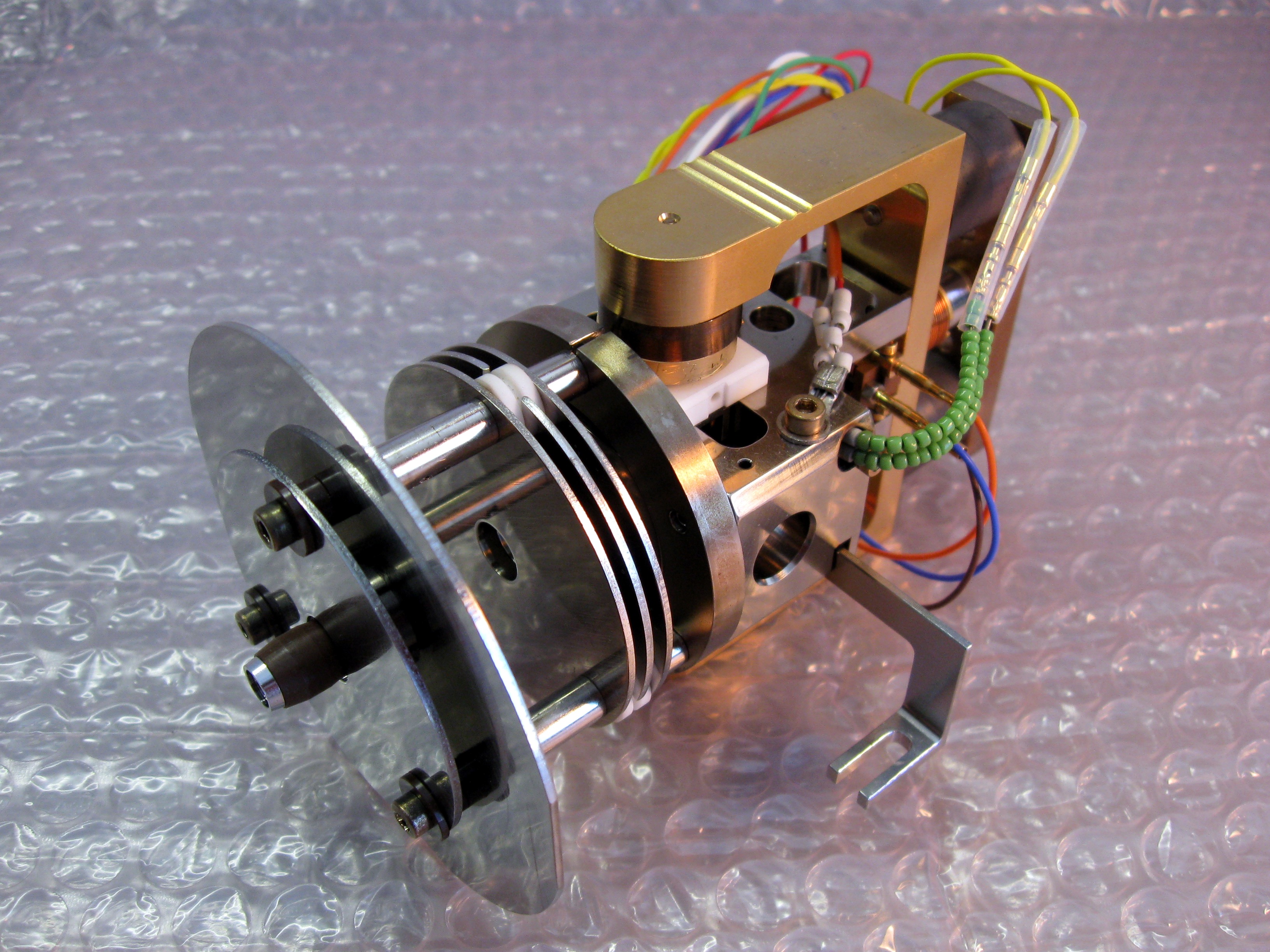
Anode Rays
An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886. Later work on anode rays by Wilhelm Wien and J. J. Thomson led to the development of mass spectrometry. Anode ray tube Goldstein used a gas-discharge tube which had a perforated cathode. When an electrical potential of several thousand volts is applied between the cathode and anode, faint luminous "rays" are seen extending from the holes in the back of the cathode. These rays are beams of particles moving in a direction opposite to the "cathode rays", which are streams of electrons which move toward the anode. Goldstein called these positive rays ''Kanalstrahlen'', "channel rays", or "canal rays", because these rays passed through the holes or ''channels'' in the cathode. The process by which anode rays are formed in a gas-discharge anode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anode Ray Tube
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode (while discharging). In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy vacuum ultraviolet, ultraviolet part of the electromagnetic spectrum are ionizing radiation; whereas the lower energy ultraviolet, visible light, infrared, microwaves, and radio waves are non-ionizing radiation. Nearly all types of laser light are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area cannot be sharply defined, as different molecules and atoms ionize at Ionization energies of the elements (data page), different energies. The energy of ionizing radiation starts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
German Inventions
German inventions and discoveries are ideas, objects, processes or techniques Invention, invented, innovated or Discovery (observation), discovered, partially or entirely, by Germans. Often, things discovered for the first time are also called inventions and in many cases, there is no clear line between the two. Germany has been the home of many List of German inventors and discoverers, famous inventors, discoverers and engineers, including Carl von Linde, who developed the modern refrigerator. Ottomar Anschütz and the Max Skladanowsky, Skladanowsky brothers were early pioneers of History of film, film technology, while Paul Gottlieb Nipkow, Paul Nipkow and Karl Ferdinand Braun laid the foundation of the television with their Nipkow disk and cathode-ray tube (or Braun tube) respectively. Hans Geiger was the creator of the Geiger counter and Konrad Zuse built the first fully automatic digital computer (Z3 (computer), Z3) and the first commercial computer (Z4 (computer), Z4). Such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emil Rupp
Philipp Heinrich Emil Rupp (1 July 1898 – 10 April 1979) was a German physicist, regarded by many as a respectable and important experimentalist in the late 1920s.Jeroen van Dongen: Emil Rupp, Albert Einstein and the Canal Ray Experiments on Wave–Particle Duality: Scientific Fraud and Theoretical Bias. In: Historical Studies in the Physical and Biological Sciences 37 Suppl. (2007), 73–120/ref> He was later forced to recant all five of the papers he had published in 1935, admitting that his findings and experiments had been fictions. There is evidence that most if not all of his earlier experimental results were forged as well. Canal ray experiments In 1926 Rupp's anode ray, canal ray experiments seemed to corroborate Albert Einstein's theories on wave–particle duality. He published these results in a paper that was printed next to a theoretical paper on the same subject by Einstein, who evidently accepted Rupp's alleged findings as confirming his (Einstein's) theoretical mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Academies
A national academy is an organizational body, usually operating with state financial support and approval, that co-ordinates scholarly research activities and standards for academic disciplines, and serves as a public policy advisors, research institutes, think tanks, and public administration consultants for governments or on issues of public importance, most frequently in the sciences but also in the humanities. Typically the country's learned societies in individual disciplines will liaise with or be coordinated by the national academy. National academies play an important organisational role in academic exchanges and collaborations between countries. The extent of official recognition of national academies varies between countries. In some cases they are explicitly or de facto an arm of government; in others, as in the United Kingdom, they are voluntary, non-profit bodies with which the government has agreed to negotiate, and which may receive government financial support while ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth Metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactivity (chemistry), reactive metals at standard temperature and pressure. Together with helium, these elements have in common an outer atomic orbital, s orbital which is full —that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with electric charge, charge +2, and an oxidation state of +2. Helium is grouped with the noble gases and not with the alkaline earth metals, but it is theorized to have some similarities to beryllium when forced into bonding and has sometimes been suggested to belong to group 2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised Homologous series, homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, hardness, sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and '' halite'' reflects this correlation. A halide ion is a halogen atom bearing a negative charge. The common halide anions are fluoride (), chloride (), bromide (), and iodide (). Such ions are present in many ionic halide salts. Halide minerals contain halides. All these halide anions are colorless. Halides also form covalent bonds, examples being colorless TiF4, colorless TiCl4, orange TiBr4, and brown TiI4. The heavier members TiCl4, TiBr4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines. Electron ionization Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is :M + e^- -> M^ + 2e^- where M is the atom or molecule being ionized, e^- is the electron, and M^ is the resulting ion. The electrons may be created by an arc discharge between a cathode and an anode. An electron beam ion source (EBIS) is used in atomic physics to produce highly charged ions by bombarding atoms with a powerful electron beam. Its principle of operation is shared by the electron beam ion trap. Electron capture ionization Electron capture ionization (ECI) is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A−•. The r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the atomic nucleus, nucleus, but can also refer to energy levels of nuclei or molecular vibration, vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be Quantization (physics), quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |