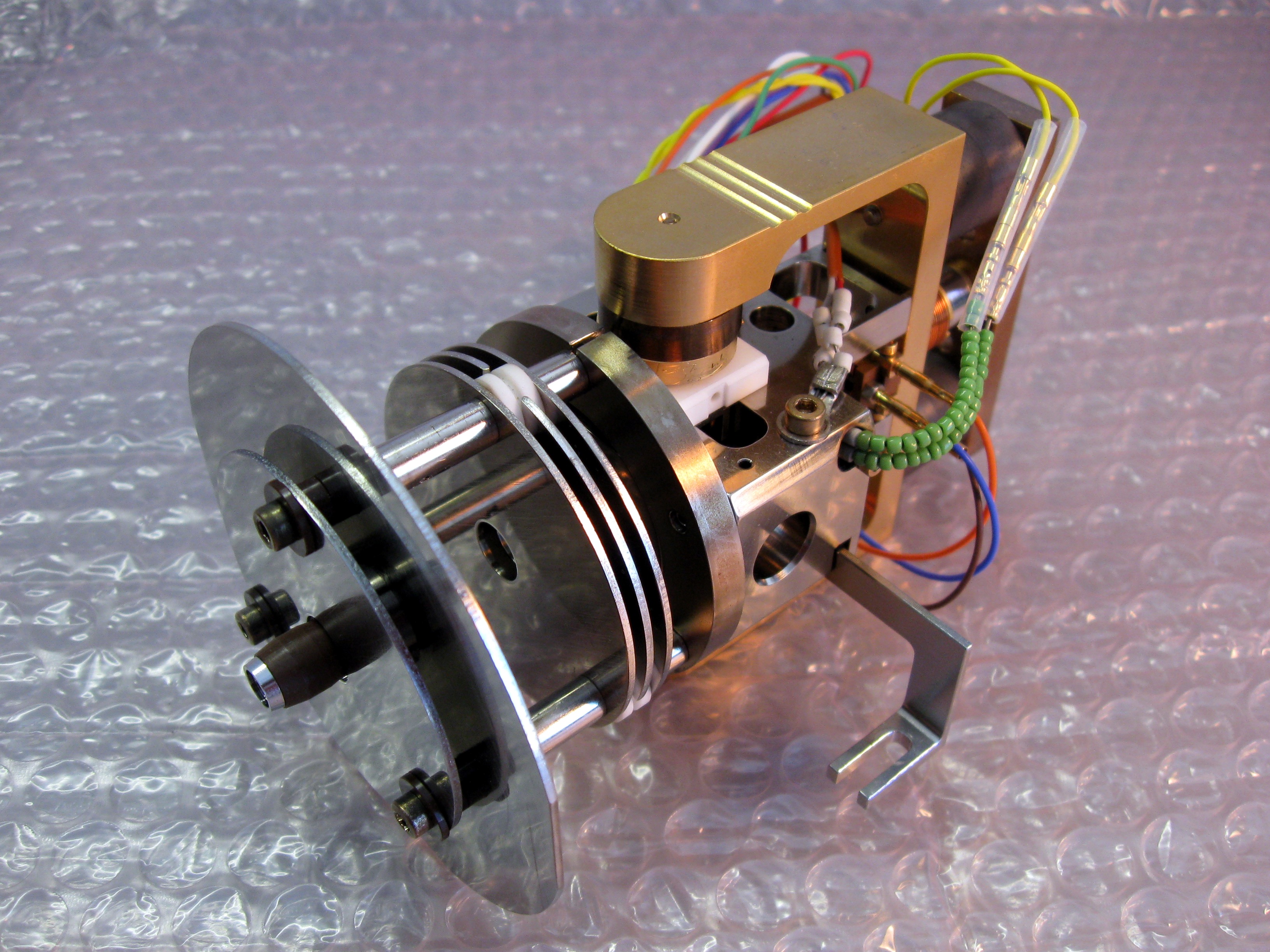
An ion source is a device that creates atomic and molecular
ions.
Ion sources are used to form
ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
for
mass spectrometers,
optical emission spectrometer
Atomic emission spectroscopy (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, Plasma (physics), plasma, Electric arc, arc, or Electric spark, spark at a particular wavelength to determine the quantity o ...
s,
particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
s,
ion implanters and
ion engines.
Electron ionization
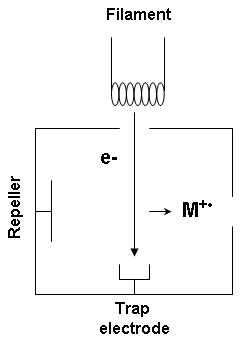 Electron ionization
Electron ionization is widely used in mass spectrometry, particularly for
organic molecules. The
gas phase reaction producing electron ionization is
:
M + e^- -> M^ + 2e^-
where M is the atom or molecule being ionized,
e^- is the electron, and
M^ is the resulting ion.
The electrons may be created by an
arc discharge between a
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
and an
anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
.
An electron beam ion source (EBIS) is used in
atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
to produce highly charged
ions by bombarding
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s with a powerful
electron beam
Since the mid-20th century, electron-beam technology has provided the basis for a variety of novel and specialized applications in semiconductor manufacturing, microelectromechanical systems, nanoelectromechanical systems, and microscopy.
Mechani ...
.
Its principle of operation is shared by the
electron beam ion trap.
Electron capture ionization
Electron capture ionization (ECI) is the ionization of a gas phase
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
or
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
by attachment of an
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
to create an ion of the form A
−•. The reaction is
:
A + e^- -> A^-
where the M over the arrow denotes that to conserve
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
and
momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
a third body is required (the
molecularity of the reaction is three).
Electron capture can be used in conjunction with
chemical ionization.
An
electron capture detector is used in some
gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t ...
systems.
Chemical ionization
Chemical ionization (CI) is a lower energy process than
electron ionization because it involves ion/molecule reactions rather than electron removal. The lower energy yields less
fragmentation, and usually a simpler
spectrum
A spectrum (: spectra or spectrums) is a set of related ideas, objects, or properties whose features overlap such that they blend to form a continuum. The word ''spectrum'' was first used scientifically in optics to describe the rainbow of co ...
. A typical CI spectrum has an easily identifiable molecular ion.
In a CI experiment, ions are produced through the collision of the analyte with ions of a
reagent gas in the ion source. Some common reagent gases include:
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
,
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, and
isobutane. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization
plasma. Positive and negative ions of the analyte are formed by reactions with this plasma. For example,
protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), usually denoted by H+, to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brø ...
occurs by
:
CH4 + e^- -> CH4+ + 2e^- (primary ion formation),
:
CH4 + CH4+ -> CH5+ + CH3 (reagent ion formation),
:
M + CH5+ -> CH4 + + H (product ion formation, e.g. protonation).
Charge exchange ionization
Charge-exchange ionization (also known as charge-transfer ionization) is a gas phase reaction between an
ion and an
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
or
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
in which the charge of the ion is transferred to the neutral species.
:
A+ + B -> A + B+
Chemi-ionization
Chemi-ionization is the formation of an
ion through the reaction of a gas phase
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
or
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
with an atom or molecule in an
excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
.
Chemi-ionization can be represented by
:
G^\ast + M -> G + M^ + e^-
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
to form the
radical cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(indicated by the superscripted "plus-dot").
Associative ionization
Associative ionization is a gas phase reaction in which two atoms or molecules interact to form a single product ion. One or both of the interacting species may have excess
internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
.
For example,
:
A^\ast + B -> AB^ + e^-
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB
+.
Penning ionization
Penning ionization is a form of chemi-ionization involving reactions between neutral atoms or molecules.
The process is named after the Dutch physicist
Frans Michel Penning who first reported it in 1927. Penning ionization involves a reaction between a gas-phase excited-state atom or molecule G
* and a target molecule M resulting in the formation of a radical molecular cation M
+., an electron e
−, and a neutral gas molecule G:
:
G^\ast + M -> G + M^ + e^-
Penning ionization occurs when the target molecule has an
ionization potential
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
lower than the internal energy of the excited-state atom or molecule.
Associative Penning ionization can proceed via
:
G^\ast + M -> MG^ + e^-
Surface Penning ionization (also known as Auger deexcitation) refers to the interaction of the excited-state gas with a bulk surface S, resulting in the release of an electron according to
:
G^\ast + S -> G + S + e^-.
Ion attachment
Ion-attachment ionization is similar to
chemical ionization in which a cation is attached to the analyte molecule in a reactive collision:
:
M + X+ + A -> MX+ + A
Where M is the analyte molecule, X
+ is the cation and A is a non-reacting collision partner.
In a radioactive ion source, a small piece of radioactive material, for instance
63 Ni or
241 Am, is used to ionize a gas. This is used in ionization
smoke detectors and
ion mobility spectrometers.
Gas-discharge ion sources

These ion sources use a
plasma source or
electric discharge to create ions.
Inductively-coupled plasma
Ions can be created in an inductively coupled plasma, which is a plasma source in which the
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
is supplied by
electrical currents which are produced by
electromagnetic induction
Electromagnetic or magnetic induction is the production of an electromotive force, electromotive force (emf) across an electrical conductor in a changing magnetic field.
Michael Faraday is generally credited with the discovery of induction in 1 ...
, that is, by time-varying
magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
s.
Microwave-induced plasma
Microwave induced plasma ion sources are capable of exciting electrodeless gas discharges to create ions for trace element mass spectrometry.
A microwave plasma has high frequency
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
in the
GHz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), often described as being equivalent to one event (or Cycle per second, cycle) per second. The hertz is an SI derived unit whose formal expression in ter ...
range. It is capable of exciting electrodeless
gas discharges. If applied in
surface-wave-sustained mode, they are especially well suited to generate large-area plasmas of high plasma density. If they are both in surface-wave and
resonator mode, they can exhibit a high degree of spatial localization. This allows to spatially separate the location of plasma generations from the location of surface processing. Such a separation (together with an appropriate gas-flow scheme) may help reduce the negative effect, that particles released from a processed substrate may have on the
plasma chemistry of the
gas phase.
ECR ion source
The ECR ion source makes use of the electron cyclotron resonance to ionize a plasma. Microwaves are injected into a volume at the frequency corresponding to the electron cyclotron resonance, defined by the magnetic field applied to a region inside the volume. The volume contains a low pressure gas.
Glow discharge

Ions can be created in an electric
glow discharge
A glow discharge is a Plasma (physics), plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a va ...
. A glow discharge is a plasma formed by the passage of electric current through a low-pressure gas. It is created by applying a voltage between two metal
electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s in an evacuated chamber containing gas. When the voltage exceeds a certain value, called the
striking voltage, the gas forms a plasma.
A
duoplasmatron is a type of glow discharge ion source that consists of a
hot cathode or
cold cathode
A cold cathode is a cathode that is not electrically heated by a Electrical filament, filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" ...
that produces a plasma that is used to ionize a gas.
THey can produce positive or negative ions.
They are used for secondary ion mass spectrometry, ion beam etching, and high-energy physics.
Flowing afterglow
In a flowing plasma afterglow, ions are formed in a flow of inert gas, typically
helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
or
argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
.
Reagents are added downstream to create ion products and study reaction rates.
Flowing-afterglow mass spectrometry is used for trace gas analysis for organic compounds.
Spark ionization
Electric spark
An electric spark is an abrupt electrical discharge that occurs when a sufficiently high electric field creates an Ionization, ionized, Electric current, electrically conductive channel through a normally-insulating medium, often air or other ga ...
ionization is used to produce gas phase
ions from a solid sample. When incorporated with a mass spectrometer the complete instrument is referred to as a spark ionization mass spectrometer or as a spark source mass spectrometer (SSMS).
A closed drift ion source uses a radial magnetic field in an annular cavity in order to confine electrons for ionizing a gas. They are used for
ion implantation and for space propulsion (
Hall-effect thrusters).
Photoionization
Photoionization is the ionization process in which an ion is formed from the interaction of a
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
with an atom or molecule.
Multi-photon ionization
In multi-photon ionization (MPI), several photons of energy below the ionization threshold may actually combine their energies to ionize an atom.
Resonance-enhanced multiphoton ionization (REMPI) is a form of MPI in which one or more of the photons accesses a
bound-bound transition that is
resonant in the atom or molecule being ionized.
Atmospheric pressure photoionization
Atmospheric pressure photoionization (APPI) uses a source of photons, usually a vacuum UV (VUV) lamp, to ionize the analyte with single photon ionization process. Analogous to other atmospheric pressure ion sources, a spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius) and sprayed with high flow rates of nitrogen for desolvation. The resulting
aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
is subjected to UV radiation to create ions.
Atmospheric-pressure laser ionization uses UV laser light sources to ionize the analyte via MPI.
Desorption ionization
Field desorption

Field desorption refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed. This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations and less often, protonated molecules
Particle bombardment
Fast atom bombardment
Particle bombardment with atoms is called fast atom bombardment (FAB) and bombardment with atomic or molecular ions is called
secondary ion mass spectrometry (SIMS).
Fission fragment ionization uses ionic or neutral atoms formed as a result of the
nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
of a suitable
nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
, for example the
Californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
isotope
252Cf.
In FAB the analytes is mixed with a non-volatile chemical protection environment called a
matrix
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the m ...
and is bombarded under vacuum with a high energy (4000 to 10,000
electron volts) beam of atoms.
The atoms are typically from an inert gas such as
argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
or
xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
. Common matrices include
glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
,
thioglycerol,
3-nitrobenzyl alcohol (3-NBA),
18-crown-6 ether,
2-nitrophenyloctyl ether,
sulfolane,
diethanolamine, and
triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
Secondary ionization
Secondary ion mass spectrometry (SIMS) is used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm.
In a
liquid metal ion source (LMIS), a metal (typically
gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
) is heated to the liquid state and provided at the end of a capillary or a needle. Then a
Taylor cone is formed under the application of a strong electric field. As the cone's tip get sharper, the electric field becomes stronger, until ions are produced by field evaporation. These ion sources are particularly used in
ion implantation or in
focused ion beam instruments.
Plasma desorption ionization
Plasma desorption ionization mass spectrometry (PDMS), also called fission fragment ionization, is a mass spectrometry technique in which ionization of material in a solid sample is accomplished by bombarding it with ionic or neutral atoms formed as a result of the
nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
of a suitable
nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
, typically the
californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
isotope
252Cf.
Laser desorption ionization

Matrix-assisted laser desorption/ionization (MALDI) is a soft ionization technique. The sample is mixed with a matrix material. Upon receiving a laser pulse, the matrix absorbs the laser energy and it is thought that primarily the matrix is desorbed and ionized (by addition of a proton) by this event. The analyte molecules are also desorbed. The matrix is then thought to transfer proton to the analyte molecules (e.g., protein molecules), thus charging the analyte.
Surface-assisted laser desorption/ionization
Surface-assisted laser desorption/ionization (SALDI) is a
soft laser desorption technique used for analyzing
biomolecule
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids ...
s by
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
.
In its first embodiment, it used
graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
matrix.
At present, laser desorption/ionization methods using other
inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
matrices, such as
nanomaterials, are often regarded as SALDI variants. A related method named "ambient SALDI" - which is a combination of conventional SALDI with ambient mass spectrometry incorporating the
DART ion source - has also been demonstrated.
Surface-enhanced laser desorption/ionization
Surface-enhanced laser desorption/ionization (SELDI) is a variant of MALDI that is used for the analysis of
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proporti ...
s that uses a target modified to achieve biochemical
affinity
Affinity may refer to:
Commerce, finance and law
* Affinity (law), kinship by marriage
* Affinity analysis, a market research and business management technique
* Affinity Credit Union, a Saskatchewan-based credit union
* Affinity Equity Pa ...
with the analyte compound.
Desorption ionization on silicon
Desorption ionization on silicon (DIOS) refers to laser desorption/ionization of a sample deposited on a porous silicon surface.
Smalley source
A laser vaporization cluster source produces ions using a combination of laser desorption ionization and supersonic expansion.
The Smalley source (or Smalley cluster source) was developed by
Richard Smalley at
Rice University
William Marsh Rice University, commonly referred to as Rice University, is a Private university, private research university in Houston, Houston, Texas, United States. Established in 1912, the university spans 300 acres.
Rice University comp ...
in the 1980s and was central to the discovery of
fullerenes in 1985.
Aerosol ionization
In
aerosol mass spectrometry
Aerosol mass spectrometry is the application of mass spectrometry to the analysis of the composition of aerosol particles. Aerosol particles are defined as solid and liquid particles suspended in a gas (air), with size range of 3 nm to 100 ...
with time-of-flight analysis, micrometer sized solid aerosol particles extracted from the atmosphere are simultaneously desorbed and ionized by a precisely timed laser pulse as they pass through the center of a time-of-flight ion extractor.
Spray ionization

Spray ionization methods involve the formation of aerosol particles from a liquid
solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI.
Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity.
The inlet tube to the mass spectrometer becomes the ion source.
Matrix-Assisted Ionization
Matrix-Assisted Ionization (MAI) is similar to MALDI in sample preparation, but a laser is not required to convert analyte molecules included in a matrix compound into gas-phase ions. In MAI, analyte ions have charge states similar to electrospray ionization but obtained from a solid matrix rather than a solvent. No voltage or laser is required, but a laser can be used to obtain spatial resolution for imaging. Matrix-analyte samples are ionized in the vacuum of a mass spectrometer and can be inserted into the vacuum through an atmospheric pressure inlet. Less volatile matrices such as 2,5-dihydroxybenzoic acid require a hot inlet tube to produce analyte ions by MAI, but more volatile matrices such as 3-nitrobenzonitrile require no heat, voltage, or laser. Simply introducing the matrix-analyte sample to the inlet aperture of an atmospheric pressure ionization mass spectrometer produces abundant ions. Compounds at least as large as bovine serum albumin
6 kDacan be ionized with this method.
In this method, the inlet to the mass spectrometer can be considered the ion source.
Atmospheric-pressure chemical ionization
Atmospheric-pressure chemical ionization uses a solvent spray at atmospheric pressure.
A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen and the entire aerosol cloud is subjected to a
corona discharge that creates ions with the evaporated solvent acting as the chemical ionization reagent gas. APCI is not as "soft" (low fragmentation) an ionization technique as ESI.
Note that atmospheric pressure ionization (API) should not be used as a synonym for APCI.
Thermospray ionization
Thermospray ionization is a form of atmospheric pressure ionization in
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
. It transfers ions from the liquid phase to the gas phase for analysis. It is particularly useful in
liquid chromatography-mass spectrometry.

Electrospray ionization
In electrospray ionization, a liquid is pushed through a very small, charged and usually metal,
capillary
A capillary is a small blood vessel, from 5 to 10 micrometres in diameter, and is part of the microcirculation system. Capillaries are microvessels and the smallest blood vessels in the body. They are composed of only the tunica intima (the inn ...
. This liquid contains the substance to be studied, the
analyte, dissolved in a large amount of
solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
, which is usually much more
volatile than the analyte. Volatile acids, bases or buffers are often added to this solution as well. The analyte exists as an
ion in solution either in its anion or cation form. Because like charges repel, the liquid pushes itself out of the capillary and forms an aerosol, a mist of small droplets about 10
μm across. The aerosol is at least partially produced by a process involving the formation of a
Taylor cone and a jet from the tip of this cone. An uncharged carrier gas such as
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
is sometimes used to help
nebulize the liquid and to help evaporate the neutral solvent in the droplets. As the solvent evaporates, the analyte molecules are forced closer together, repel each other and break up the droplets. This process is called Coulombic fission because it is driven by repulsive
Coulombic forces between charged molecules. The process repeats until the analyte is free of solvent and is a bare ion. The ions observed are created by the addition of a
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
(a hydrogen ion) and denoted , or of another
cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
such as sodium ion, , or the removal of a proton, . Multiply charged ions such as are often observed. For
macromolecules, there can be many charge states, occurring with different frequencies; the charge can be as great as , for example.
Probe electrospray ionization
Probe electrospray ionization (PESI) is a modified version of electrospray, where the capillary for sample solution transferring is replaced by a sharp-tipped solid needle with periodic motion.
Contactless atmospheric pressure ionization
Contactless atmospheric pressure ionization is a technique used for analysis of liquid and solid samples by mass spectrometry.
Contactless API can be operated without an additional electric power supply (supplying voltage to the source emitter), gas supply, or
syringe pump. Thus, the technique provides a facile means for analyzing chemical compounds by mass spectrometry at atmospheric pressure.
Sonic spray ionization
Sonic spray ionization is method for creating ions from a liquid solution, for example, a mixture of methanol and water.
A
pneumatic nebulizer is used to turn the solution into a
supersonic spray of small droplets. Ions are formed when the solvent evaporates and the statistically unbalanced charge distribution on the droplets leads to a net charge and complete desolvation results in the formation of ions. Sonic spray ionization is used to analyze small organic molecules and drugs and can analyze large molecules when an electric field is applied to the capillary to help increase the charge density and generate multiple charged ions of proteins.
Sonic spray ionization has been coupled with
high performance liquid chromatography for the analysis of drugs.
Oligonucleotides have been studied with this method.
SSI has been used in a manner similar to desorption electrospray ionization
for
ambient ionization and has been coupled with
thin-layer chromatography in this manner.
Ultrasonication-assisted spray ionization
Ultrasonication-assisted spray ionization (UASI) is similar to the above techniques but uses an ultrasonic transducer to achieve atomization of the material and generate ions.
Thermal ionization
Thermal ionization (also known as surface ionization, or contact ionization) involves spraying vaporized, neutral atoms onto a hot surface, from which the atoms re-evaporate in ionic form. To generate positive ions, the atomic species should have a low
ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
, and the surface should have a high
work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
. This technique is most suitable for
alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
atoms (Li, Na, K, Rb, Cs) which have low ionization energies and are easily evaporated.
To generate negative ions, the atomic species should have a high
electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
This differs by si ...
, and the surface should have a low work function. This second approach is most suited for
halogen atoms Cl, Br, I, At.
Ambient ionization

In ambient ionization, ions are formed outside the mass spectrometer without sample preparation or separation.
Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by
chemical ionization, or laser
desorbed or
ablated and post-ionized before they enter the mass spectrometer.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface.
Molecules on the surface are extracted into the solvent. The action of the primary droplets hitting the surface produces secondary droplets that are the source of ions for the mass spectrometer. Desorption electrospray ionization (DESI) creates charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.
Plasma-based ambient ionization is based on an electrical discharge in a flowing gas that produces metastable atoms and molecules and reactive ions. Heat is often used to assist in the desorption of volatile species from the sample. Ions are formed by chemical ionization in the gas phase. A
direct analysis in real time (DART) source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or
"metastables").
Excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
s are typically formed in the DART source by creating a glow discharge in a chamber through which the gas flows. A similar method called atmospheric solids analysis probe (ASAP) uses the heated gas from ESI or APCI probes to vaporize sample placed on a melting point tube inserted into an ESI/APCI source.
Ionization is by APCI.
Laser-based ambient ionization is a two-step process in which a pulsed laser is used to desorb or ablate material from a sample and the plume of material interacts with an electrospray or plasma to create ions. Electrospray-assisted laser desorption/ionization (ELDI) uses a 337 nm UV laser
or 3 μm infrared laser
to desorb material into an electrospray source.
Matrix-assisted laser desorption electrospray ionization (MALDESI) is an atmospheric pressure ionization source for generation of multiply charged ions. An ultraviolet or infrared laser is directed onto a solid or liquid sample containing the analyte of interest and matrix desorbing neutral analyte molecules that are ionized by interaction with electrosprayed solvent droplets generating multiply charged ions.
Laser ablation electrospray ionization (LAESI) is an ambient ionization method for mass spectrometry that combines laser ablation from a mid-infrared (mid-IR) laser with a secondary
electrospray ionization (ESI) process.
Applications
Mass spectrometry
In a mass spectrometer a sample is ionized in an ion source and the resulting ions are separated by their mass-to-charge ratio. The ions are detected and the results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio. The atoms or molecules in the sample can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern.
Particle accelerators


In particle accelerators an ion source creates a
particle beam at the beginning of the machine, the ''source''. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated:
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s,
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s,
H− ion or a
Heavy ions.
Electrons are generated with an
electron gun, of which there are many varieties.
Protons are generated with a
plasma-based device, like a
duoplasmatron or a
magnetron
The cavity magnetron is a high-power vacuum tube used in early radar systems and subsequently in microwave oven, microwave ovens and in linear particle accelerators. A cavity magnetron generates microwaves using the interaction of a stream of ...
.
H− ions are generated with a
magnetron
The cavity magnetron is a high-power vacuum tube used in early radar systems and subsequently in microwave oven, microwave ovens and in linear particle accelerators. A cavity magnetron generates microwaves using the interaction of a stream of ...
or a
Penning
Penning may refer to:
__NOTOC__ Currency
*Norwegian penning
*Swedish penning
People
*Mike Penning (born 1957), British politician
*Frans Michel Penning (1894–1953), Dutch physicist
*Edmund Penning-Rowsell (1913–2002), British journalist
*Lou ...
source. A magnetron consists of a central cylindrical cathode surrounded by an anode. The discharge voltage is typically greater than 150 V and the current drain is around 40 A. A
magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
of about 0.2
tesla is parallel to the
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
axis. Hydrogen gas is introduced by a pulsed gas valve.
Caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
is often used to lower the
work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
of the cathode, enhancing the amount of ions that are produced. Large caesiated sources are also used for
plasma heating in nuclear fusion devices.
For a
Penning source, a strong magnetic field parallel to the electric field of the sheath guides electrons and ions on cyclotron spirals from cathode to cathode. Fast H-minus ions are generated at the cathodes as in the magnetron. They are slowed down due to the charge exchange reaction as they migrate to the plasma aperture. This makes for a beam of ions that is colder than the ions obtained from a magnetron.
Heavy ions can be generated with an
electron cyclotron resonance ion source. The use of electron cyclotron resonance (ECR) ion sources for the production of intense beams of highly charged ions has immensely grown over the last decade. ECR ion sources are used as injectors into linear accelerators, Van-de-Graaff generators or cyclotrons in nuclear and elementary particle physics. In atomic and surface physics ECR ion sources deliver intense beams of highly charged ions for collision experiments or for the investigation of surfaces. For the highest charge states, however,
Electron beam ion sources (EBIS) are needed. They can generate even bare ions of mid-heavy elements. The
Electron beam ion trap (EBIT), based on the same principle, can produce up to bare uranium ions and can be used as an ion source as well.
Heavy ions can also be generated with an
ion gun which typically uses the thermionic emission of electrons to ionize a substance in its gaseous state. Such instruments are typically used for surface analysis.

Gas flows through the ion source between the anode and the cathode. A positive
voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
is applied to the anode. This voltage, combined with the high magnetic field between the tips of the internal and external cathodes allow a plasma to start. Ions from the plasma are repelled by the anode's electric field. This creates an ion beam.
Surface modification
* Surface cleaning and pretreatment for large area deposition
*
Thin film
A thin film is a layer of materials ranging from fractions of a nanometer ( monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many ...
deposition
* Deposition of thick
diamond-like carbon (DLC) films
* Surface roughening of
polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, b ...
for improved
adhesion
Adhesion is the tendency of dissimilar particles or interface (matter), surfaces to cling to one another. (Cohesion (chemistry), Cohesion refers to the tendency of similar or identical particles and surfaces to cling to one another.)
The ...
and/or
biocompatibility
See also
*
Ion beam
*
RF antenna ion source
*
On-Line Isotope Mass Separator
References
{{authority control
Ions
Accelerator physics
 An ion source is a device that creates atomic and molecular ions. Ion sources are used to form
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form  Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is
:
Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is
: These ion sources use a plasma source or electric discharge to create ions.
These ion sources use a plasma source or electric discharge to create ions.
 Ions can be created in an electric
Ions can be created in an electric  Field desorption refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed. This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations and less often, protonated molecules
Field desorption refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed. This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations and less often, protonated molecules
 Spray ionization methods involve the formation of aerosol particles from a liquid solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI. Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity. The inlet tube to the mass spectrometer becomes the ion source.
Spray ionization methods involve the formation of aerosol particles from a liquid solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI. Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity. The inlet tube to the mass spectrometer becomes the ion source.

 In ambient ionization, ions are formed outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface. Molecules on the surface are extracted into the solvent. The action of the primary droplets hitting the surface produces secondary droplets that are the source of ions for the mass spectrometer. Desorption electrospray ionization (DESI) creates charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.
Plasma-based ambient ionization is based on an electrical discharge in a flowing gas that produces metastable atoms and molecules and reactive ions. Heat is often used to assist in the desorption of volatile species from the sample. Ions are formed by chemical ionization in the gas phase. A direct analysis in real time (DART) source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or "metastables").
In ambient ionization, ions are formed outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface. Molecules on the surface are extracted into the solvent. The action of the primary droplets hitting the surface produces secondary droplets that are the source of ions for the mass spectrometer. Desorption electrospray ionization (DESI) creates charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.
Plasma-based ambient ionization is based on an electrical discharge in a flowing gas that produces metastable atoms and molecules and reactive ions. Heat is often used to assist in the desorption of volatile species from the sample. Ions are formed by chemical ionization in the gas phase. A direct analysis in real time (DART) source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or "metastables"). 
 In particle accelerators an ion source creates a particle beam at the beginning of the machine, the ''source''. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated:
In particle accelerators an ion source creates a particle beam at the beginning of the machine, the ''source''. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated: