|
Andersonite
Andersonite, Na2Ca(UO2)(CO3)3·6H2O, or hydrated sodium calcium uranyl carbonate is a rare uranium carbonate mineral that was first described in 1948. Named after Charles Alfred Anderson (1902–1990) of the United States Geological Survey, who first described the mineral species, it is found in sandstone-hosted uranium deposits. It has a high vitreous to pearly Lustre (mineralogy), luster and is fluorescent. Andersonite specimens will usually glow a bright lemon yellow (or green with blue hints depending on the deposit) in ultraviolet light. It is commonly found as translucent small rhombohedral crystals that have angles close to 90 degrees although its crystal system is nominally trigonal. Its Mohs scale of mineral hardness, Mohs hardness is 2.5, with an average specific gravity of 2.8. It occurs in the Redox, oxidized zone of uranium-bearing polymetallic ore deposits. It also may occur as an efflorescent crust on the walls and timbers of uranium mines. As this mineral is wate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 **Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite (mineral), Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Alfred Anderson
Charles Alfred "Andy" Anderson (June 6, 1902January 9, 1990) was an American geologist. He was the chief geologist of the United States Geological Survey from 1959 to 1964. Early life Anderson attended Pomona College, graduating in 1924. He earned his doctorate from the University of California, Berkeley in 1928. Career Anderson taught at UC Berkeley for 14 years. He began a career with the United States Geological Survey The United States Geological Survey (USGS), founded as the Geological Survey, is an agency of the U.S. Department of the Interior whose work spans the disciplines of biology, geography, geology, and hydrology. The agency was founded on Mar ... in 1942, and was its chief geologist from 1959 to 1964. Anderson's 50-year career in geoscience research on ore deposits, volcanic rocks and Precambrian geology included 30 years with the USGS and 20 years associated with the University of California. In honor of his contributions to the knowledge of mineral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohs Scale Of Mineral Hardness
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material. The scale was introduced in 1812 by the German geologist and mineralogist Friedrich Mohs, in his book (English: Attempt at an elementary method for the natural-historical determination and recognition of fossils); it is one of several definitions of hardness in materials science, some of which are more quantitative. The method of comparing hardness by observing which minerals can scratch others is of great antiquity, having been mentioned by Theophrastus in his treatise ''On Stones'', , followed by Pliny the Elder in his ''Naturalis Historia'', . The Mohs scale is useful for identification of minerals in the field, but is not an accurate predictor of how well materials endure in an industrial setting. Reference minerals The Mohs scale of mineral hardness is based on the ability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also Crystallization, crystallizes as translucent crystals of selenite (mineral), selenite. It forms as an evaporite mineral and as a Mineral hydration, hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on Scratch hardness, scratch hardness comparison. Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Etymology and history The word ''wikt:gypsum, gypsum'' is derived from the Greek language, Greek word (), "plaster". Because the quarry, quarries of the Montmartre district of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liebigite
Liebigite is a uranium carbonate mineral with the chemical formula: Ca2(UO2)(CO3)3·11H2O. It is a secondary mineral occurring in the oxidizing zone of uranium-bearing ores. It is green to yellow green in colour. It has a Mohs hardness of about 3. Liebigite, like some other uranium minerals, is fluorescent under UV light and is also translucent. It crystallizes in the orthorhombic system, but only rarely forms distinct crystals. It typically forms encrustations or granular aggregates. It was first described in 1848 for an occurrence in Adrianople, Edirne Province, Marmara Region, Turkey. It was named for German chemist Justus von Liebig Justus ''Freiherr'' von Liebig (12 May 1803 – 18 April 1873) was a Germans, German scientist who made major contributions to the theory, practice, and pedagogy of chemistry, as well as to agricultural and biology, biological chemistry; he is ... (1803–1873). References Carbonate minerals Uranium(VI) minerals Orthorhombic miner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltwoodite
Boltwoodite is a hydrated uranyl silicate mineral with formula (K0.56Na0.42) UO2)(SiO3OH)�1.5(H2O), distinct in crystal structure from sodium boltwoodite, which has an orthorhombic structure rather than monoclinic. It is formed from the oxidation and alteration of primary uranium ores. It takes the form of a crust on some sandstones that bear uranium. These crusts tend to be yellowish with a silky or vitreous luster. Discovery and occurrence It was first described in 1956 for an occurrence in Pick's Delta Mine, Delta, San Rafael District (San Rafael Swell), Emery County, Utah, US. It is named after Bertram Boltwood (1870–1927) an American pioneer of radiochemistry. Boltwoodite occurs as secondary silicate alteration crusts surrounding uraninite and as fracture fillings. It is found in pegmatites and sandstone uranium deposits of the Colorado Plateau-type. It occurs associated with uraninite, becquerelite, fourmarierite, phosphouranylite, gypsum and fluorite Fluorite (als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bayleyite
Bayleyite is a uranium carbonate mineral with the chemical formula: Mg2(UO2)(CO3)3·18(H2O). It is a secondary mineral which contains magnesium, uranium and carbon. It is a bright yellow color. Its crystal habit is acicular but is more commonly found as crusts on uranium bearing ores. It has a Mohs hardness of about 2–2.5. Occurrence It was first described in 1948 for an occurrence in the Hillside mine, north of Bagdad, Yavapai County, Arizona and named for mineralogist William Shirley Bayley (1861–1943) of the University of Illinois. It occurs as an efflorescence or coating on other secondary minerals and often is deposited on mine walls and workings. It occurs with schrockingerite, andersonite, swartzite and gypsum in the Hillside mine; with schrockingerite and gypsum in the Hideout mine in Utah; and with tyuyamunite, uranophane, liebigite and carnotite in the Powder River Basin in Wyoming Wyoming ( ) is a landlocked U.S. state, state in the Mountain states, Mou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
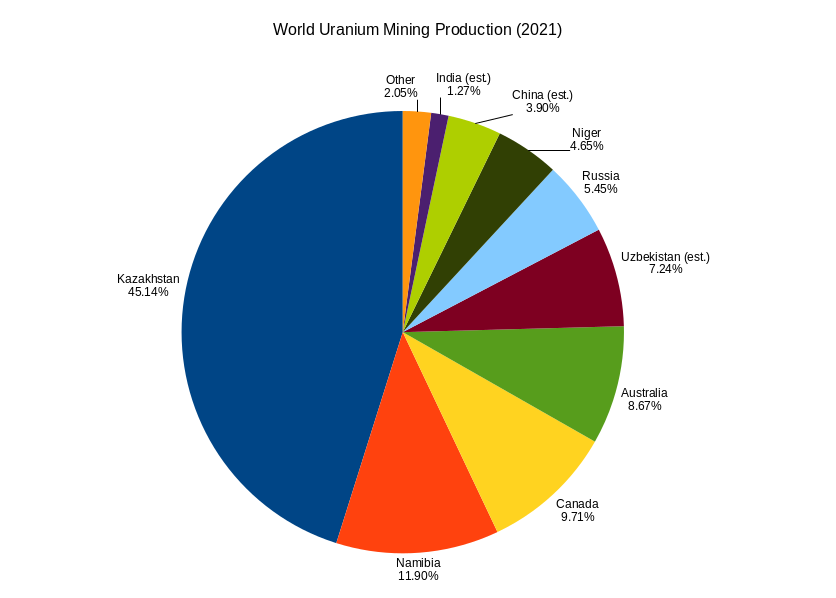
Uranium Mine
Uranium mining is the process of extraction of uranium ore from the earth. Over 50,000 tons of uranium were produced in 2019. Kazakhstan, Canada, and Australia were the top three uranium producers, respectively, and together account for 68% of world production. Other countries producing more than 1,000 tons per year included Namibia, Niger, Russia, Uzbekistan and China. Nearly all of the world's mined uranium is used to power nuclear power plants. Historically uranium was also used in applications such as uranium glass or ferrouranium but those applications have declined due to the radioactivity and toxicity of uranium and are nowadays mostly supplied with a plentiful cheap supply of depleted uranium which is also used in uranium ammunition. In addition to being cheaper, depleted uranium is also less radioactive due to a lower content of short-lived and than natural uranium. Uranium is mined by in-situ leaching (57% of world production) or by conventional underground or op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efflorescent
In chemistry, efflorescence (Derived from the Latin verb 'efflorescere' roughly meaning 'to flower') is the migration of a salt to the surface of a porous material, where it forms a coating. The essential process involves the dissolving of an internally held salt in water or occasionally, in another solvent. The water, with the salt now held in solution, migrates to the surface, then evaporates, leaving a coating of the salt. In what has been described as "primary efflorescence", the water is the invader and the salt was already present internally, and a reverse process, where the salt is originally present externally and is then carried inside in solution, is referred to as "secondary efflorescence". Efflorescences can occur in natural and built environments. On porous construction materials it may present a cosmetic outer problem only (primary efflorescence causing staining), but can sometimes indicate internal structural weakness (migration/degradation of component materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |