|
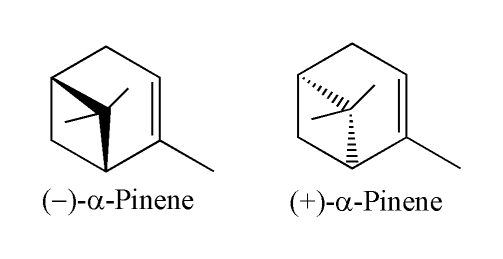
Alicyclic Compound
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. Cycloalkanes The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include decalin, housane, and norbornane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, and so on. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornane
Norbornane (also known as bicyclo .2.1eptane) is an organic compound and a saturated hydrocarbon with chemical formula C7H12. It is a crystalline compound with a melting point of 88 °C. The carbon skeleton is derived from cyclohexane ring with a methylene bridge in the 1,4- position, and is a bridged bicyclic compound. The compound is a prototype of a class of strained bicyclic hydrocarbons. The compound was originally synthesized by reduction of norcamphor. The name norbornane is derived from bornane, which is 1,7,7-trimethylnorbornane, being a derivative of camphor (bornanone). The prefix ''nor'' refers to the stripping of the methyl groups from the parent molecule bornane. See also * 2-Norbornyl cation * Norbornene * Norbornadiene * Bornane * endo-Norborneol * exo-Norborneol * Norcamphor Norcamphor is an organic compound, classified as a bicyclic ketone. It is an structural analog, analog of camphor, but without the three methyl groups. A colorless solid, it is u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutene
Cyclobutene is an organic compound with the chemical formula . It is a cycloalkene. It is a colorless gas that easily condenses. It is of interest in research but currently has no practical applications. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared by thermolysis of the ammonium salt (cyclobutyltrimethylammonium hydroxide). Cyclobutene thermally isomerizes to 1,3-butadiene. This strongly exothermic reaction reflects the dominance of ring strain. In contrast, the corresponding equilibrium for hexafluorocyclobutene disfavors hexafluorobutadiene. See also * Cyclobutane Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ... * Cyclobutadiene * Cyclobutyne * Squaric acid References {{cycloalkenes Monomers Cycloa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit. Structure and bonding The molecule has a triangular structure. The reduced length of the double bond compared to a single bond causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbon atoms use sp2.68 hybridization for the ring. Synthesis of cyclopropene and derivatives Early syntheses The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkene
In organic chemistry, a cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed Ring (chemistry), ring of carbon atoms and either one or more double bonds, but has no Aromaticity, aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the Cis–trans isomerism, ''cis'' isomers, and the term ''cis'' tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond; however, this is not feasible in smaller molecules due to the increase of Ring strain, strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene Structures
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an intermediate in the commercial synthesis of nylon. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. The main product of the process is cyclohexane because cyclohexene is more easily hydrogenated than benzene. In the laboratory, it can be prepared by dehydration of cyclohexanol. : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst. 1,5-Hexadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leopold Ruzicka
Leopold may refer to: People * Leopold (given name), including a list of people named Leopold or Léopold * Leopold (surname) Fictional characters * Leopold (''The Simpsons''), Superintendent Chalmers' assistant on ''The Simpsons'' * Leopold Bloom, the protagonist of James Joyce's ''Ulysses'' * Leopold "Leo" Fitz, on the television series ''Agents of S.H.I.E.L.D.'' * Leopold "Butters" Stotch, on the television series ''South Park'' * General Leopold von Flockenstuffen, on the BBC sitcom Allo 'Allo!'' * Leopold the Cat, the protagonist of a Soviet/Russian animated short film series * Leopold, 3rd Duke of Albany, a lead character of '' Kate & Leopold'', a 2001 romantic comedy film * Leopold Slikk, an alias of Norman Kochanowski known for Angry German Kid Businesses *Leopold (publisher), a Netherlands-based publishing company *Leopold Bros., an American micro-distiller * Leopold Cafe, Colaba, Mumbai, India (attacked during the 26 November 2008 Mumbai attacks) * Leopold's Ic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature, peace, and physiology or medicine. This award is administered by the Nobel Foundation and awarded by the Royal Swedish Academy of Sciences on proposal of the Nobel Committee for Chemistry, which consists of five members elected by the Academy. The award is presented in Stockholm at an annual ceremony on December 10th, the anniversary of Nobel's death. The first Nobel Prize in Chemistry was awarded in 1901 to Jacobus Henricus van 't Hoff, of the Netherlands, "for his discovery of the laws of chemical dynamics and osmotic pressure in solutions". From 1901 to 2024, the award has been bestowed on a total of 195 individuals. The 2024 Nobel Prize in Chemistry was awarded to Demis Hassabis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Wallach
Otto Wallach (; 27 March 1847 – 26 February 1931) was a German chemist and recipient of the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Biography Wallach was born in Königsberg, the son of a Prussian civil servant. His father, Gerhard Wallach, descended from a Jewish family that had converted to Lutheranism. His mother, Otillie (Thoma), was an ethnic German of Protestant religion. Wallach's father was transferred to Stettin (Szczecin) and later to Potsdam. Otto Wallach went to school, a ''Gymnasium'', in Potsdam, where he learned about literature and the history of art, two subjects he was interested his whole life. At this time he also started private chemical experiments at the house of his parents. In 1867 he started studying chemistry at the University of Göttingen, where at this time Friedrich Wöhler was head of organic chemistry. After one semester at the University of Berlin with August Wilhelm von Hofmann, Wallach received his Doctoral degree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baldwin's Rules
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin (chemist), Jack Baldwin in 1976. Baldwin's rules discuss the relative rates of ring closures of these various types. These terms are not meant to describe the absolute probability that a reaction will or will not take place, rather they are used in a relative sense. A reaction that is disfavoured (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favoured (fast). However, the disfavoured product may be observed, if no alternate reactions are more favoured. The rules classify ring closures in three ways: *the number of atoms in the newly formed ring *into ''exo'' and ''endo'' ring closures, depending whether the bond broken during the ring closure is inside (''endo'') or outside (''exo'') the ring that is being formed *into ''tet'', ''trig'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are Organic compound, compounds that have at least two Cyclic compound, molecular rings sharing one common atom. Simple spiro compounds are bicyclic (having just two rings). The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone. The common atom that connects the two (or sometimes three) rings is called the ''spiro atom''. In carbocyclic spiro compounds like spiro[5.5]undecane, the spiro-atom is a quaternary carbon, and as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
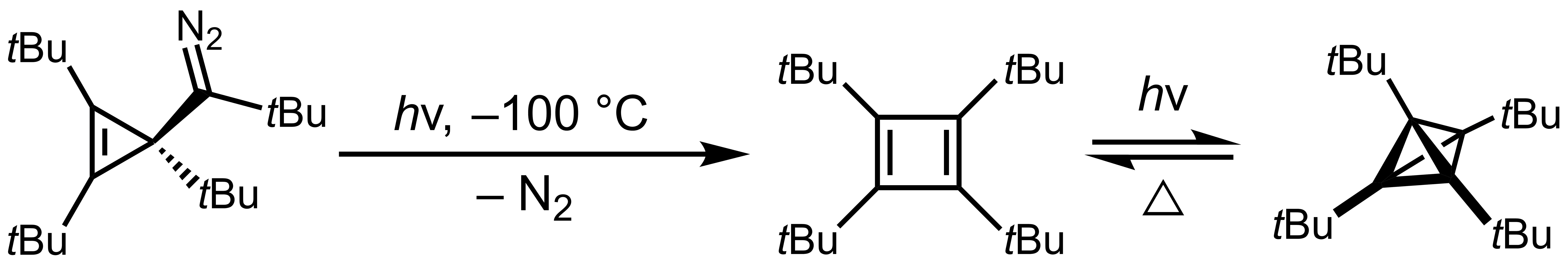
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. C4 tetrahedranes Tetrahedrane () is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane remains elusive, although predicted kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon. Contrariwise, several organic compounds with the tetrahedrane core are known. All have multiply bulky substituents, ''tert''-butyl (''t''-Bu) or larger. Locking a tetrahedrane molecule inside a fullerene has only been attempted ''in silico''. All known syntheses have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |