|
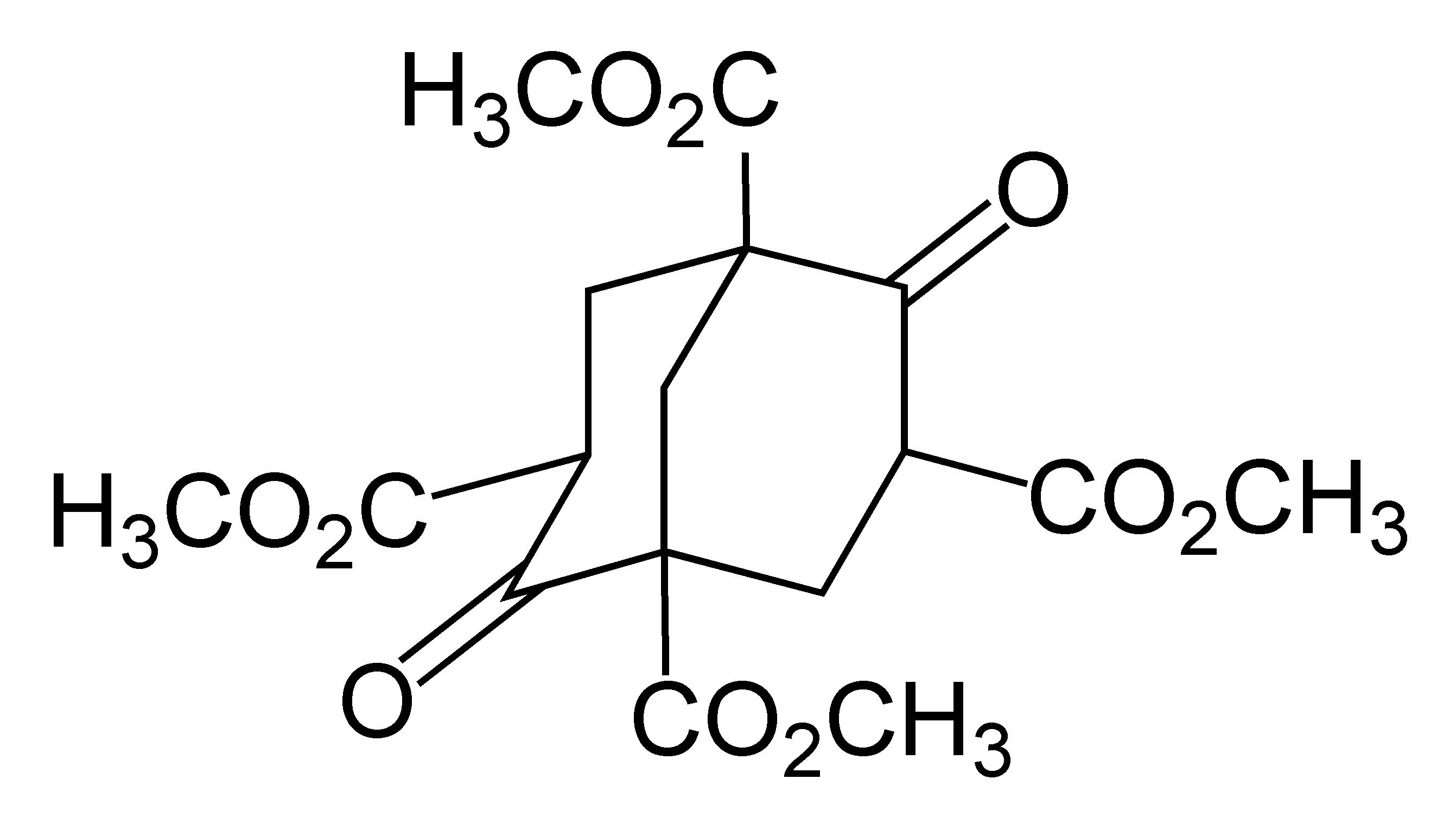
Adamantane Dication
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans Meerwein
Hans Meerwein (May 20, 1879 in Hamburg, Germany – October 24, 1965 in Marburg, Germany) was a German chemist. Several reactions and reagents bear his name, most notably the Meerwein–Ponndorf–Verley reduction, the Wagner–Meerwein rearrangement, the Meerwein arylation reaction, and Meerwein's salt. Life and work His father was the architect, Wilhelm Emil Meerwein. He originally trained to be a chemistry technician or 'chemotechnician' at the Fresenius University of Applied Sciences (between 1898 and 1900) before studying for a chemistry degree at the University of Bonn. After finishing his PhD with Richard Anschütz he worked at the University of Berlin, before returning to Bonn where he became professor in 1914. From 1922 till 1928 he was professor for organic chemistry at the University of Königsberg. The last change in his academic career was to the University of Marburg. The war devastated the Institute and Meerwein was planning the rebuilding which was finis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adams' Catalyst
Adams Pearmain, also called Adam's Parmane, is a cultivar of apple. It was introduced to the Royal Horticultural Society, Horticultural Society of London in 1826 by Robert Adams, under the name Norfolk Pippin. The fruit is large, varying from two and a half inches to three inches high, and about the same in breadth at the widest part. It is pearmain-shaped, very even, and regularly formed. The skin is pale yellow tinged with green, and covered with delicate russet on the shaded side; but deep yellow tinged with red, and delicately streaked with livelier red on the side facing the sun. The flesh is reddish, crisp, juicy, rich, and sugary, with an agreeable and pleasantly perfumed flavor.The Fruit Manual, Hogg This Cultivar is a sibling of Reinette de Hollande, a hybrid between Reinette Franche’ and ‘Reinette des Carmes. (5) See also * Pearmain Notes References 5: Muranty, H., Denancé, C., Feugey, L., Crépin, J. L., Barbier, Y., Tartarini, S., ... Durel, C. E. (2020). Us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula . At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints. The top seven suppliers worldwide together had an annual capacity in 2001 of 179 kilotonnes (395 million pounds). DCPD was discovered in 1885 as a hydrocarbon among the products of pyrolysis of phenol by Henry Roscoe, who didn't identify the structure (that was made during the following decade) but accurately assumed that it was a dimer of some hydrocarbon. History and structure For many years the structure of dicy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Von Ragué Schleyer
Paul von Ragué Schleyer (February 27, 1930 – November 21, 2014) was an American physical organic chemist whose research is cited with great frequency. A 1997 survey indicated that Dr. Schleyer was, at the time, the world's third most cited chemist, with over 1100 technical papers produced. He was Eugene Higgins Professor of Chemistry at Princeton University, professor and co-director of the Institute for Organic Chemistry (Institut für organische Chemie) at the University of Erlangen–Nuremberg in Germany, and later Graham Perdue Professor of Chemistry at the University of Georgia in Athens, Georgia, Athens, Georgia (U.S. state), Georgia. He published twelve books in the fields of lithium chemistry, Ab initio quantum chemistry methods, ab initio molecular orbital theory and carbonium ions. He was past president of the World Association of Theoretically Oriented Chemists, a fellow of the International Academy of Quantum Molecular Science and editor-in-chief of the ''Encycloped ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: : Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Overall, decarboxylation depends upon stability of the carbanion synthon , although the anion may not be a true chemical intermediate. Typically, carboxylic acids decarboxylate slowly, but carboxylic acids with an α el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adamantane Synthesis By Prelog
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vladimir Prelog
Vladimir Prelog (23 July 1906 – 7 January 1998) was a Croatian-Swiss organic chemist who received the 1975 Nobel Prize in chemistry for his research into the stereochemistry of organic molecules and reactions. Prelog was born, and spent his infancy, in Sarajevo, and youth in Zagreb, Osijek and Prague.Vladimir Prelog (1975Autobiography the Nobel Committee. He later lived and worked in Prague, Zagreb and Zürich. Early life Prelog was born in Sarajevo, Condominium of Bosnia and Herzegovina, at that time within Austria-Hungary, to Croat parents who were working there. His father, Milan, a native of Zagreb, was a history professor at a gymnasium in Sarajevo and later at the University of Zagreb. As an 8-year-old boy, he stood near the place where the assassination of Franz Ferdinand occurred. Education Prelog started elementary school in Sarajevo. In 1915, at the beginning of the first World War, at Prelog moved to Zagreb (then part of Austria-Hungary) with his parents. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Millions of tonnes are produced annually, mainly as a precursor to nylon. History and synthesis The compound was discovered by in 1888 among the products of AC electrolysis of slightly acidified water solutions of phenol. He named it hydrophenoketone and correctly suggested that phenol was first hydrogenated by electrolytic hydrogen to cyclohexanol, which he wasn't able to isolate, and then oxidized by electrolytic oxygen. Laboratory synthesis Cyclohexanone can be prepared from cyclohexanol by oxidation with chromium trioxide ( Jones oxidation). An alternative method utilizes the safer and more readily avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phloroglucinol
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the organic synthesis, synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "natural phenol, phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed polyphenols. The enzyme is biosynthesized by phloroglucinol synthase. Synthesis and occurrence In 1855, phloroglucinol was first prepared from phloretin by the Austrian chemist Heinrich Hlasiwetz (1825–1875). A modern synthesis of phloroglucinol involves hydrolysis of benzene-1,3,5-triamine and its derivatives. Representative is the following route from 1,3,5-Trinitrobenzene, trinitrobenzene. : The synthesis is noteworthy because ordinary aniline deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |