|
Acetyltransferase
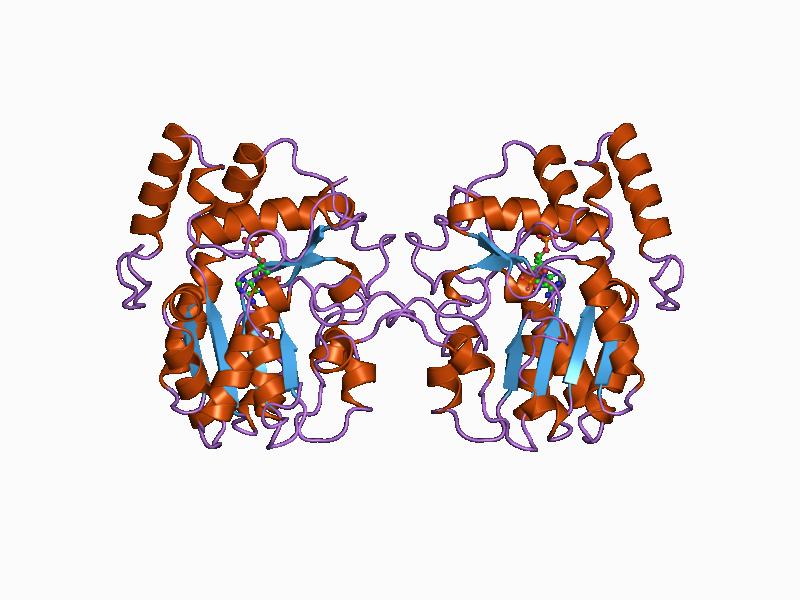
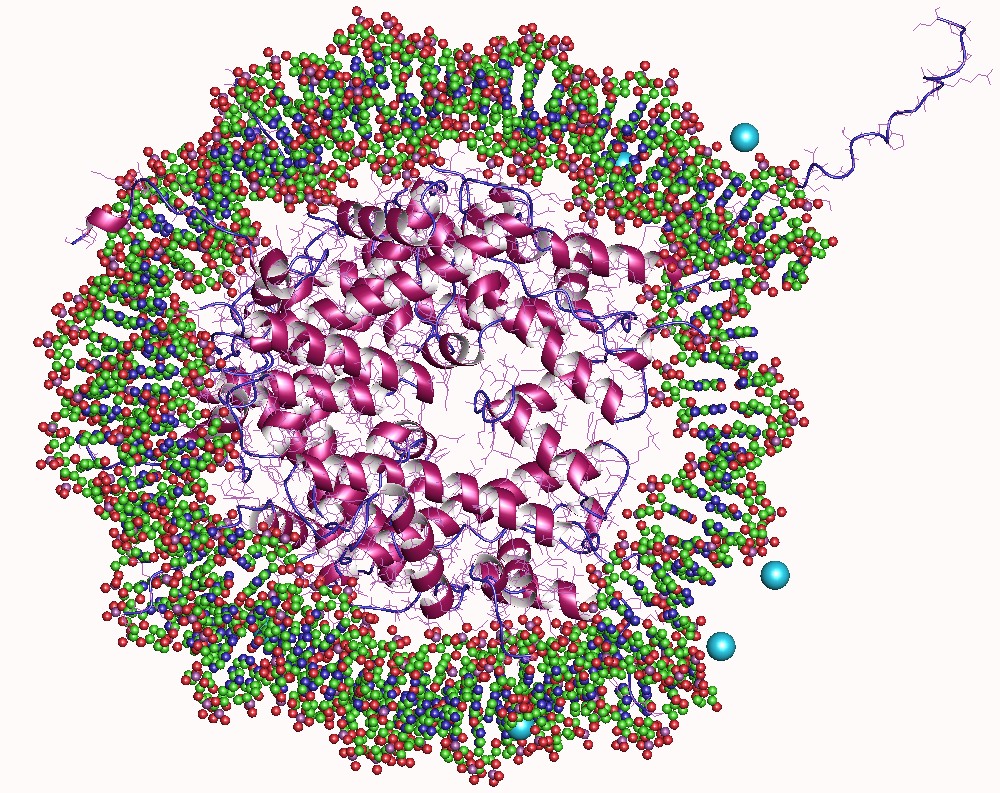
An acetyltransferase (also referred to as a transacetylase) is any of a class of transferase enzymes that transfers an acetyl group in a reaction called acetylation. In biological organisms, post-translational modification of a protein via acetylation can profoundly transform its functionality by altering various properties like hydrophobicity, solubility, and surface attributes. These alterations have the potential to influence the protein's conformation and its interactions with substrates, cofactors, and other macromolecules. Types of acetyltransferases Additional examples of acetyltransferases found in nature include: * Chloramphenicol acetyltransferase Structure The predicted three-dimensional structures of histone, choline, and serotonin acetyltransferases are shown below. As with all enzymes, the structures of acetyltransferases are essential for interactions between them and their substrates; alterations to the structures of these enzymes often result in a loss o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
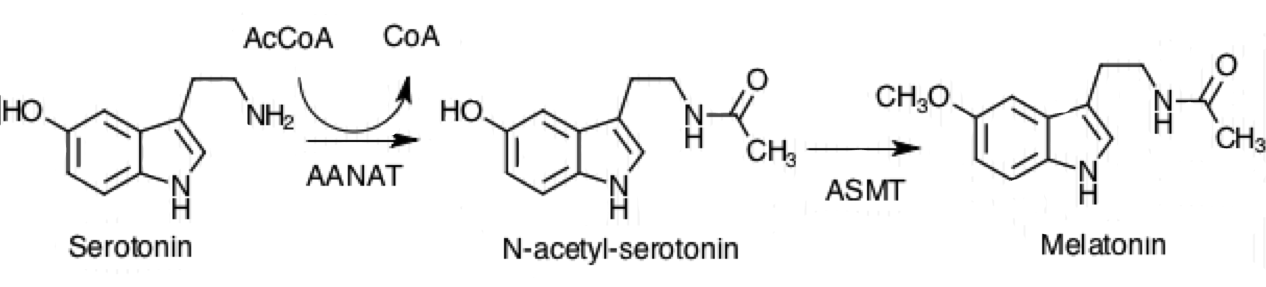
Serotonin N-acetyltransferase
Aralkylamine ''N''-acetyltransferase (AANAT) (), also known as arylalkylamine ''N''-acetyltransferase or serotonin ''N''-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb '' AANAT gene'' containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of ''N''-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function. Nomenclature The systematic name of this enzyme class is acetyl-CoA:2-arylethylamine N-acetyltransferase. Other names in common use include: * AANAT * Arylalkylamin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Acetyltransferase
Histone acetyltransferases (HATs) are enzymes that acetylation, acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine, ε-''N''-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression. In general, histone acetylation is linked to DNA transcription, transcriptional activation and associated with euchromatin. Euchromatin, which is less densely compact, allows transcription factors to bind more easily to regulatory sites on DNA, causing transcriptional activation. When it was first discovered, it was thought that acetylation of lysine neutralizes the positive electric charge, charge normally present, thus reducing affinity between histone and (negatively charged) DNA, which renders DNA more accessible to transcription factors. Research has emerged, since, to show that lys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Choline Acetyltransferase
Choline acetyltransferase (commonly abbreviated as ChAT, but sometimes CAT) is a transferase enzyme responsible for the synthesis of the neurotransmitter acetylcholine. ChAT catalyzes the transfer of an acetyl group from the coenzyme acetyl-CoA to choline, yielding acetylcholine (ACh). ChAT is found in high concentration in cholinergic neurons, both in the central nervous system (CNS) and peripheral nervous system (PNS). As with most nerve terminal proteins, ChAT is produced in the body of the neuron and is transported to the nerve terminal, where its concentration is highest. Presence of ChAT in a nerve cell classifies this cell as a "cholinergic" neuron. In humans, the choline acetyltransferase enzyme is encoded by the ''CHAT'' gene. History Choline acetyltransferase was first described by David Nachmansohn and A. L. Machado in 1943. A German biochemist, Nachmansohn had been studying the process of nerve impulse conduction and utilization of energy-yielding chemical react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GCN5
__NOTOC__ Histone acetyltransferase KAT2A is an enzyme that in humans is encoded by the ''KAT2A'' gene. Interactions GCN5L2 has been shown to interact with: * DDB1, * Ku70, * Ku80, * TADA2L, * TAF9, and * Transcription initiation protein SPT3 homolog Transcription initiation protein SPT3 homolog is a protein that in humans is encoded by the ''SUPT3H'' gene. Interactions Transcription initiation protein SPT3 homolog has been shown to interact with GCN5L2, TAF6L, TADA3L, TAF5L, SF3B3, .... References Further reading * * * * * * * * * * * * * * * * * * External links * {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramphenicol Acetyltransferase
Chloramphenicol acetyltransferase (or CAT) is a bacterial enzyme () that detoxifies the antibiotic chloramphenicol and is responsible for chloramphenicol resistance in bacteria. This enzyme covalently attaches an acetyl group from acetyl-CoA to chloramphenicol, which prevents chloramphenicol from binding to ribosomes. A histidine residue, located in the C-terminal section of the enzyme, plays a central role in its catalytic mechanism. The crystal structure of the type III enzyme from ''Escherichia coli'' with chloramphenicol bound has been determined. CAT is a trimer of identical subunits (monomer Mr 25,000) and the trimeric structure is stabilised by a number of hydrogen bonds, some of which result in the extension of a beta-sheet across the subunit interface. Chloramphenicol binds in a deep pocket located at the boundary between adjacent subunits of the trimer, such that the majority of residues forming the binding pocket belong to one subunit while the catalytically essential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NatA Acetyltransferase
NatA acetyltransferase(Nα acetyltransferase), is an enzyme that serves to catalyze the addition of acetyl groups to various proteins emerging from the ribosome. Upon translation, the NatA binds to the ribosome and then "stretches" to the front end of the forming, or nascent, polypeptide, where it adds this acetyl group. This acetyl group is added to the front end, or N-terminus of the new protein. Forty percent of all proteins in the yeast proteome are thought to be N-terminally acetylated, with a corresponding figure of 90% in mammalian proteins. To be specific, NatA is the main N-terminal acetyltransferase in the yeast cytosol, responsible for the acetylation of proteins at locations in which L-serine, L-alanine, L-threonine, or glycine are present. NatA Acetyltransferase is not a single protein but a complex of three subunits. Sup35p acetylation In ''Saccharomyces cerevisiae'' NatA acetyltransferase interacts with the Sup35p protein. It is involved in the reaction of the SI+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histones
In biology, histones are highly Base (chemistry), basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei and in most Archaea, Archaeal Phylum, phyla. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 9 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones, which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B protein dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl group is called an ethanoyl group. An acetyl group contains a methyl group () that is single-bonded to a carbonyl (), making it an acyl group. The carbonyl center of an acyl radical has one non-bonded electron with which it forms a chemical bond to the remainder (denoted with the letter ''R'') of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation Acetylation is the chemical reaction known as "ethanoylation" in the IUPAC nomenclature. It depicts a reactionary process that injects an acetyl functional group into a chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NatB Acetyltransferase
NatB acetyltransferase is an enzyme in the ''Saccharomyces cerevisiae'' that functions to catalyze the dehydration synthesis of the addition of an acetyl group onto a nascent polypeptide. A list of 18 proteins are known to be acetylated by this enzyme. The subclasses of proteins with abundances of the amino acids aspartic acid, methionine Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ... specifically are acetylated. {{Acyltransferases Saccharomycetes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylation
: In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Acetylation/deacetylation in biology Histone deacetylases "play crucial roles in gene transcription and most likely in all eukaryotic biological processes that involve chromatin". Acetylation is one type of post-translational modification of proteins. The acetylation of the ε-amino group of lysine, which is common, converts a charged side chain to a neutral one. Acetylation/deacetylation of histones also plays a role in gene expression and cancer. These modifications are effected by enzymes called histone acetyltransferases (HATs) and histone deacetylases (HDACs). Two general mechanisms are known for deacetylation. One mechanism involves zinc binding to the acetyl o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |