|
AM-1235
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1. Pharmacology Pharmacodynamics AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2. While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times. This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2. Pharmacokinetics AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 ''N''- dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors. Pharmacology AM-694 is an agonist for cannabinoid receptors. It has a ''K''i of 0.08 nM at CB1 and 18 times selectivity over CB2 with a ''K''i of 1.44 nM. It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body. Metabolism Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the ''N''-alkyl chain. See also * AM-679 * AM-1235 * AM-2201 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
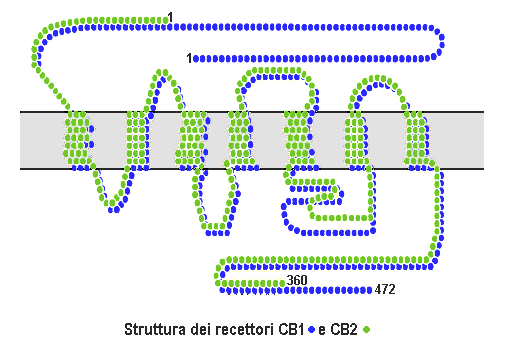
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: * Endocannabinoids; * Phytocannabinoids (plant-derived such as tetrahydrocannabinol (THC) produced by cannabis); * Synthetic cannabinoids (such as HU-210). All endocannabinoids and phytocannabinoids are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2232
AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a ''K''i of 0.28 nM at CB1 and 1.48 nM at CB2. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-2232 are Schedule I Controlled Substances. See also * AM-694 * AM-1235 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * O-774 * O-1057 * O-1812 * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor, CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring h ... References Naphthoylindoles Nitriles AM cannabinoids Designer drugs {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2389
AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a ''K''i of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action. Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2. High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812 O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a ''K''i of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1812
O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a ''K''i of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes. See also * AM-1235 * AM-2232 AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a ''K''i of 0.28 nM at CB1 and 1.48 nM at CB2. In the United States, all CB1 receptor ... * AM-2389 * Methanandamide * O-774 * O-1057 References Cannabinoids Nitriles Fatty acid amides {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor, CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been replaced by indazole. Pharmacology THJ-2201 acts as a full agonist with a Affinity (pharmacology)#Protein-ligand binding, binding affinity of 1.34 nM at Cannabinoid receptor type 1, CB1 and 1.32 nM at Cannabinoid receptor type 2, CB2 cannabinoid receptors. Side effects THJ-2201 has been linked to at least one hospitalization and death due to its use. Legal status It is classified as a Schedule I controlled substance in the United States. It is also an Drugs controlled by the German Betäubungsmittelgesetz, Anlage II controlled drug in Germany. See also * AM-694 * AM-1235 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * NM-2201 * THJ-018 References {{Phenethylamines C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1221
AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a ''K''i of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-1221 are Schedule I Controlled Substances. Legal status It is illegal to supply, trade, sell, distribute, import or transport the pharmaceutical drug in the UK under the Psychoactive Substances Act 2016 which was in force on May 26, 2016. See also * AM-630 * AM-1220 * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University. Hazards Convulsions have been reported including at doses as low as 10 mg. Pharmacology AM-2201 is a full agonist for cannabinoid receptors. Affinities are: with a ''K''i of 1.0 nM at CB1 and 2.6 nM at CB2. The 4-methyl functional analog MAM-2201 probably has similar affinities. AM-2201 has an EC50 of 38 nM for human CB1 receptors, and 58 nM for human CB2 receptors. AM-2201 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, comparable to the potency of JWH-018 in rats, suggesting potent cannabinoid-like activity. Pharmacokinetics AM-2201 metabolism differs only slightly from that of JWH-018. AM-2201 ''N''- dealkylation produces fluoropentane instead o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor Type 2
The cannabinoid receptor 2 (CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of cannabinoids on the immune sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 1
Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild dagga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activates the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol. The primary endogenous agonist of the human CB1 receptor is ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |