|
6-Hydroxy-DMT
6-Hydroxy-DMT, also known as 6-hydroxy-''N'',''N''-dimethyltryptamine, is a serotonin receptor modulator of the tryptamine family. "6-HO-DMT is a minor metabolite of DMT in man, and it was studied for the same reasons. Could this compound play a role in explaining the activity of the parent dialkylamine? It was explored in a series of subjects who had responded spectacularly to DMT. The five volunteers in this study were former opium addicts who were serving sentences for violation of United States narcotics laws. They were administered 6-HO-DMT at either 0.75 mg/kg (one subject) or 1.0 mg/kg (four subjects) and reported no differences from the inactive placebo control. The objective measures (blood pressure, respiration and heart rate, pupillary dilation) confirmed this absence of activity at this level. The active control drug was DMT itself, and it showed the expected responses in all regards." .."It is pretty generally accepted that 6-HO-DMT is inactive. I am not too surprise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
13-Hydroxy-LSD
13-Hydroxy-LSD is a lysergamide and a metabolite of the psychedelic drug lysergic acid diethylamide (LSD). It is a major metabolite of LSD in rats and guinea pigs but a minor metabolite of LSD in monkeys and humans. Following its formation, 13-hydroxy-LSD undergoes further metabolism via glucuronidation. Little is known about the specific enzymes responsible for generation of LSD metabolites such as 13-hydroxy-LSD in humans. According to David E. Nichols in 2016, the pharmacology of hydroxylated metabolites of LSD like 13-hydroxy-LSD has not been studied. Nichols has posited that metabolism of LSD into active metabolites with potent dopamine receptor activity may be responsible for the delayed-onset dopaminergic stimulus effects of LSD in rodent drug discrimination tests. Relatedly, lergotrile's corresponding metabolite 13-hydroxylergotrile is several-fold more potent as a dopamine receptor agonist than lergotrile itself ''in vitro''. However, more research is needed to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Tryptamine
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5,6-MDO-DMT
5,6-MDO-DMT, or 5,6-methylenedioxy-''N'',''N''-dimethyltryptamine, is a lesser-known psychedelic drug. It is the 5,6-methylenedioxy analog of DMT. 5,6-MDO-DMT was first synthesized by Alexander Shulgin. In his book ''TiHKAL'' (''Tryptamines I Have Known and Loved''), 5,6-MDO-DMT produces no noticeable psychoactive effects, although it was only tested up to a dose of 5 mg. Very little data exists about the pharmacological properties, metabolism, and toxicity of 5,6-MDO-DMT. See also * Tryptamine * Dimethyltryptamine * 5,6-MDO-MiPT * 6-Hydroxy-DMT 6-Hydroxy-DMT, also known as 6-hydroxy-''N'',''N''-dimethyltryptamine, is a serotonin receptor modulator of the tryptamine family. "6-HO-DMT is a minor metabolite of DMT in man, and it was studied for the same reasons. Could this compound play a ... External links 5,6-MDO-DMT Entry in ''TIHKAL''5,6-MDO-DMT Entry in TiHKAL • info N,N-Dialkyltryptamines Dimethylamino compounds Methylenedioxytryptamines {{Psychoa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-MeO-DMT
6-MeO-DMT, or 6-methoxy-''N'',''N''-dimethyltryptamine, also known as 6-OMe-DMT, is a serotonergic drug of the tryptamine family. It is the 6-methoxy derivative of the serotonergic psychedelic ''N'',''N''-dimethyltryptamine (DMT) and is a positional isomer of the psychedelic 5-MeO-DMT. Similarly to analogues like DMT and 5-MeO-DMT, 6-MeO-DMT acts as a serotonin 5-HT2A receptor agonist as well as a non-selective agonist of many other serotonin receptors. However, in contrast to these agents, but similarly to certain other serotonin 5-HT2A receptor agonists like 6-fluoro-DET, 2-bromo-LSD, lisuride, 25N-N1-Nap, and tabernanthalog, 6-MeO-DMT does not produce the head-twitch response (HTR) or other psychedelic-like effects in animals and hence appears to be non-hallucinogenic. Similarly, 6-MeO-DMT failed to substitute for DOM in rodent drug discrimination tests. On the other hand, it did substitute for the atypical psychedelic 5-MeO-DMT in rodent drug discrimination tests, wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-Hydroxytryptamine
6-Hydroxytryptamine (6-HT or 6-HO-T) is a serotonin receptor modulator of the substituted tryptamine, tryptamine family related to serotonin. It is a positional isomer of serotonin (5-hydroxytryptamine; 5-HT) and of 4-hydroxytryptamine (4-HT). Pharmacology 6-Hydroxytryptamine shows dramatically reduced affinity (pharmacology), affinity for serotonin receptors, including the serotonin 5-HT1A receptor, 5-HT1A, 5-HT1B receptor, 5-HT1B, 5-HT2A receptor, 5-HT2A, and 5-HT2C receptor, 5-HT2C receptors (Ki = 1,590nM, 5,890nM, 11,500nM, and 5,500nM, respectively), compared to serotonin, 4-hydroxytryptamine, 5-methoxytryptamine, and certain other tryptamines. It did not produce hyperlocomotion in rodents but did partially reverse reserpine-induced hypoactivity. 6-Hydroxytryptamine appears to be less susceptible to drug metabolism, metabolism by monoamine oxidase (MAO) than serotonin. History 6-Hydroxytryptamine was first described in the scientific literature by the 1950s. Derivatives Cer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Tryptamine
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
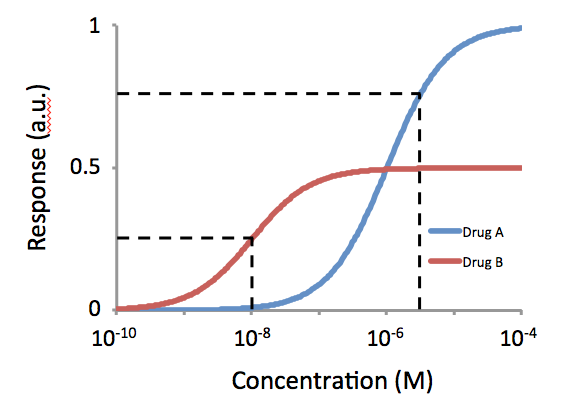
Potency (pharmacology)
In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug (e.g., fentanyl, clonazepam, risperidone, benperidol, bumetanide) evokes a given response at low concentrations, while a drug of lower potency (e.g. morphine, alprazolam, ziprasidone, haloperidol, furosemide) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness nor more side effects nor less side effects. Types of potency The International Union of Basic and Clinical Pharmacology (IUPHAR) has stated that "potency is an imprecise term that should always be further defined", and lists of types of potency as follows: Miscellaneous Lysergic acid diethylamide (LSD) is one of the most potent psychoactive drug A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-Fluoro-DMT
6-Fluoro-DMT, also known as 6-fluoro-''N'',''N''-dimethyltryptamine, is a synthetic serotonin receptor modulator of the tryptamine family. Effects 6-Fluoro-DMT has been said to not be active as a hallucinogen in humans. It has been claimed that this is due to it being " metabolically blocked", though this was not further elaborated on. In the 1960s, it had been theorized by Stephen Szára and colleagues that psychedelic tryptamines were prodrugs that required 6-hydroxylation to become hallucinogenic, but this theory was later found to be incorrect. 6-Fluoro-DMT has been thought to be inactive as a psychedelic in part because 6-fluoro-DET is inactive in terms of such effects. Pharmacology 6-Fluoro-DMT is known to possess varying affinities for serotonin receptors, adrenergic receptors, dopamine receptors, histamine receptors, the imidazoline I1 receptor, sigma receptors, and the serotonin transporter (SERT). It has been found to be a potent partial agonist of the seroto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scientific Literature
Scientific literature encompasses a vast body of academic papers that spans various disciplines within the natural and social sciences. It primarily consists of academic papers that present original empirical research and theoretical contributions. These papers serve as essential sources of knowledge and are commonly referred to simply as "the literature" within specific research fields. The process of academic publishing involves disseminating research findings to a wider audience. Researchers submit their work to reputable journals or conferences, where it undergoes rigorous evaluation by experts in the field. This evaluation, known as peer review, ensures the quality, validity, and reliability of the research before it becomes part of the scientific literature. Peer-reviewed publications contribute significantly to advancing our understanding of the world and shaping future research endeavors. Original scientific research first published in scientific journals co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2 Receptor
The 5-HT2 receptors are a subfamily of 5-HT receptors that bind the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT2 subfamily consists of three G protein-coupled receptor G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related ...s (GPCRs) which are coupled to Gq/G11 and mediate excitatory neurotransmission, including 5-HT2A, 5-HT2B, and 5-HT2C. For more information, please see the respective main articles of the individual subtypes: * 5-HT2A receptor * 5-HT2B receptor * 5-HT2C receptor See also * 5-HT1 receptor * 5-HT3 receptor * 5-HT4 receptor * 5-HT5 receptor * 5-HT6 receptor * 5-HT7 receptor * 5-HT2 antagonists References {{Serotonergics Serotonin receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, known as clinical trials, and whole plants. Definition ''In vitro'' (Latin language, Latin for "in glass"; often not italicized in English usage) studies are conducted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |