|
5F-MPMI
5F-MPMI is a tryptamine derivative which acts as a serotonin receptor agonist, selective for the 5-HT2 subtypes but with similar affinity to all three receptors, having strongest activity at 5-HT2B and weakest at 5-HT2A. See also * 4-HO-MPMI * 5-MeO-MPMI 5-MeO-MPMI (also known as 5-Methoxy-''N''-methyl-(α,''N''-trimethylene)tryptamine) is a tryptamine derivative that is a psychedelic drug. It was first developed by the team led by JE Macor in 1992, and subsequently investigated by the team led b ... * MPMI References {{Hallucinogens Designer drugs Psychedelic tryptamines Serotonin receptor agonists Tryptamines Pyrrolidines Fluoroarenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MPMI (drug)
3-(N-methylpyrrolidin-3-ylmethyl)indole (MPMI) is a tryptamine derivative which acts as a serotonin receptor agonist. It has been studied as an analogue and trace impurity of the anti-migraine drug eletriptan but is otherwise little known. See also * α,N,N-Trimethyltryptamine * Dimethyltryptamine * 4-HO-MPMI * 5F-MPMI * 5-MeO-MPMI * NTBT * Pyr-T * SN-22 SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19nM at 5HT2 subtypes vs 514 nM at 5-HT1A receptors. Many related derivatives are known, most of which are ligand ... References Designer drugs Psychedelic tryptamines Serotonin receptor agonists Tryptamines Pyrrolidines {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
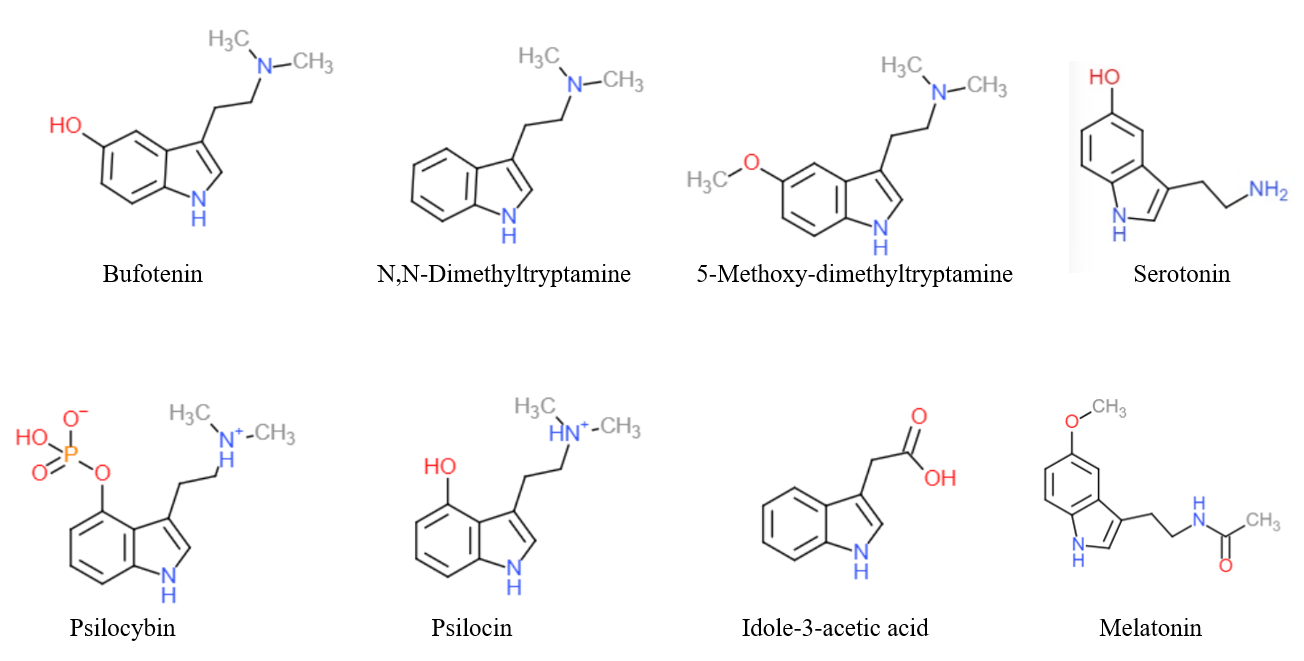
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the Central nervous system, central and peripheral nervous systems. They mediate both Neurotransmitter#Excitatory and inhibitory, excitatory and inhibitory Synaptic transmission, neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural Ligand (biochemistry), ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamic acid, glutamate, gamma-Aminobutyric acid, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, Mood (psychology), mood, nausea, sleep, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2 Receptor
The 5-HT2 receptors are a subfamily of 5-HT receptors that bind the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT2 subfamily consists of three G protein-coupled receptors (GPCRs) which are coupled to Gq/G11 and mediate excitatory neurotransmission Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), ..., including 5-HT2A, 5-HT2B, and 5-HT2C. For more information, please see the respective main articles of the individual subtypes: * 5-HT2A receptor * 5-HT2B receptor * 5-HT2C receptor See also * 5-HT1 receptor * 5-HT3 receptor * 5-HT4 receptor * 5-HT5 receptor * 5-HT6 receptor * 5-HT7 receptor * 5-HT2 antagonists References {{Serotonergics Serotonin receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2B Receptor
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the ''HTR2B'' gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Tissue distribution and function First discovered in the stomach of rats, 5-HT2B was challenging to characterize initially because of its structural similarity to the other 5-HT2 receptors, particularly 5-HT2C. The 5-HT2 receptors (of which the 5-HT2B receptor is a subtype) mediate many of the central and peripheral physiologic functions of serotonin. Cardiovascular effects include contraction of blood vessels and shape changes in platelets; central nervous system (CNS) effects include neuronal sensitization to tactile stimuli and mediation of some of the effects of hallucinogenic substituted amphetamines. The 5-HT2B receptor is expressed in several areas of the CNS, including the dorsal hypothalamus, frontal cortex, med ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-HO-MPMI
4-HO-MPMI (also known as 4-Hydroxy-''N''-methyl-(α,''N''-trimethylene)-tryptamine or lucigenol) is a tryptamine derivative that is a psychedelic drug. It was developed by the team led by David Nichols from Purdue University in the late 1990s. This compound produces hallucinogen-appropriate responding in animal tests with a similar potency to the amphetamine- derived psychedelic DOI, and has two enantiomers, with only the (''R'')-enantiomer being active. The binding affinity for 5-HT2A receptor is 13 ± 2 nM (Ki 25IOI). It is reported at doses starting at 0.5 mg and 1.0-1.5 mg seem to be psychedelic doses. The duration it is reported between six and eight hours. The effects, still not too documented, are OEV/CEV, sedation and anxiety. See also * 4-HO-pyr-T * 4-HO-McPeT * 5-MeO-MPMI * 5-MeO-pyr-T * CP-135,807 * MPMI * SN-22 SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19nM at 5H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-MPMI
5-MeO-MPMI (also known as 5-Methoxy-''N''-methyl-(α,''N''-trimethylene)tryptamine) is a tryptamine derivative that is a psychedelic drug. It was first developed by the team led by JE Macor in 1992, and subsequently investigated by the team led by David Nichols from Purdue University in the late 1990s. This compound produces psychedelic-appropriate responding in animal tests with a similar potency to the amphetamine- derived psychedelic DOI, and has two enantiomers, with only the (''R'')-enantiomer being active. See also * 5-MeO-pyr-T * 4-HO-MPMI * 4-HO-pyr-T * CP-135,807 CP-135807 is a drug which acts as a potent and selective agonist for the 5-HT1D serotonin receptor, and is used to study the function of this receptor subtype. See also * 4-HO-MPMI * 5-MeO-MPMI * Eletriptan * LY-334370 LY-334370 is a selecti ... * MPMI References {{Tryptamines Psychedelic tryptamines Designer drugs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychedelic Tryptamines
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").Pollan, Michael (2018). ''How to Change Your Mind: What the New Science of Psychedelics Teaches Us About Consciousness, Dying, Addiction, Depression, and Transcendence'' Sometimes, they are called classic hallucinogens, serotonergic hallucinogens, or serotonergic psychedelics, and the term ''psychedelics'' is used more broadly to include all hallucinogens; this article uses the narrower definition of ''psychedelics''. Psychedelics cause specific psychological, visual, and auditory changes, and often a substantially altered state of consciousness.Leary, Timothy; Metzner, Ralph (1964). ''The Psychedelic Experience: A Manual Based on The Tibetan Book of the Dead'' Psychedelic states are often compared to meditative, psychodynamic or transcendental types of alterations of mind. The "classical" psychedelics, the psy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor Agonists
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |