|
3-Quinuclidone
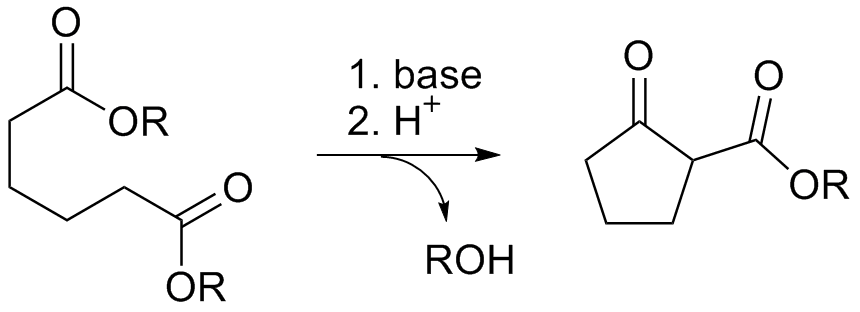
3-Quinuclidinone is a bicyclic organic compounds with chemical formula . Its basicity is indicated by the pKa of the conjugate acid, which is 7.2. In contrast quinuclidine is about 100x more basic. Synthesis and reactions Its hydrochloride salt can be synthesized by a Dieckman condensation: It is a precursor to quinuclidine. Organic reduction of 3-quinuclidone gives the compound quinuclidine, structurally related to DABCO, which has one additional bridgehead nitrogen atom. References {{DEFAULTSORT:Quinuclidone, 3- Quinuclidines Cyclic ketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinuclidine
Quinuclidine is an organic compound with the formula . It is a bicyclic amine that can be viewed as a tied back version of triethylamine. It is a colorless solid. It is used as a reagent (base) and catalyst. It can be prepared by reduction of quinuclidone. Structure and chemical properties Regarding its structure, quinuclidine is unusual in that the methylene hydrogen atoms are eclipsed within each of the three ethylene linkages. Furthermore, the cyclohexane rings, of which there are three, adopt the boat conformations, not the usual chair conformations. Quinuclidine is a relatively strong organic base with p''K''a of the conjugate acid of 11.3. The basicity of other quinuclidines have been evaluated: 3-hydroxy- quinuclidine (9.9), 3-acetoxyquinuclidine (9.3), 3-chloroquinuclidine (8.9), DABCO (8.7), and 3-quinuclidone (7.2). It forms adducts with a variety of Lewis acids. Because of its compact structure, quinuclidine binds to trimethylborane more tightly than does tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spiro compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the bridgehead atoms are directly connected (''e.g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: \text + \text \; \ce \; \text + \text Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which said that any compound that can give a proton to another compound is an acid, and the compound that receives the proton is a base. A proton is a subatomic particle in the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dieckman Condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann Walter Dieckmann (8 October 1869 – 12 January 1925) was a German chemist. He is the namesake of the Dieckmann condensation, the intramolecular reaction of diesters with base to give β-keto esters. Dieckmann studied at the University of Mun ... (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. Dieckmann condensations are highly effective routes to 5-, 6-, and 7-member rings, but poor for larger rings. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted–Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Regarding their structures, both DABCO and quinuclidine are unusual in that the methylene hydrogen atoms are eclipsed within each of the three ethylene linkages. Furthermore, the diazacyclohexane rings, of which there are three, adopt the boat conformations, not the usual chair conformations. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote a variety of coupling reactions. Catalyst DABCO is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |