|
öE (color Space)
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. For a thermodynamic process affecting a thermodynamic system without transfer of matter, the law distinguishes two principal forms of energy transfer, heat and thermodynamic work. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat transfer, thermodynamic work, and matter transfer, into and out of the system. Energy cannot be created or destroyed, but it can be transformed from one form to another. In an externally isolated system, with internal changes, the sum of all forms of energy is constant. An equivalent statement is that perpetual motion machines of the first kind are impossible; work done by a system on its surroundings requires that the system's internal energy be consumed, so that the amount of internal energy lost by that work must be resupplied as heat by an external energy s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conservation Of Energy
The law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be Conservation law, ''conserved'' over time. In the case of a Closed system#In thermodynamics, closed system, the principle says that the total amount of energy within the system can only be changed through energy entering or leaving the system. Energy can neither be created nor destroyed; rather, it can only be transformed or transferred from one form to another. For instance, chemical energy is Energy conversion, converted to kinetic energy when a stick of dynamite explodes. If one adds up all forms of energy that were released in the explosion, such as the kinetic energy and potential energy of the pieces, as well as heat and sound, one will get the exact decrease of chemical energy in the combustion of the dynamite. Classically, the conservation of energy was distinct from the conservation of mass. However, special relativity shows that mass is related to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and light. Energy is a Conservation law, conserved quantityãthe law of conservation of energy states that energy can be Energy transformation, converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J). Forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a Classical field theory, field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, the internal energy contained within a thermodynamic system, and rest energy associated with an object's rest mass. These are not mutual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
George H
George may refer to: Names * George (given name) * George (surname) People * George (singer), American-Canadian singer George Nozuka, known by the mononym George * George Papagheorghe, also known as Jorge / GEûRGE * George, stage name of Giorgio Moroder * George, son of Andrew I of Hungary Places South Africa * George, South Africa, a city ** George Airport United States * George, Iowa, a city * George, Missouri, a ghost town * George, Washington, a city * George County, Mississippi * George Air Force Base, a former U.S. Air Force base located in California Computing * George (algebraic compiler) also known as 'Laning and Zierler system', an algebraic compiler by Laning and Zierler in 1952 * GEORGE (computer), early computer built by Argonne National Laboratory in 1957 * GEORGE (operating system), a range of operating systems (George 1ã4) for the ICT 1900 range of computers in the 1960s * GEORGE (programming language), an autocode system invented by Charles L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primitive Notion
In mathematics, logic, philosophy, and formal systems, a primitive notion is a concept that is not defined in terms of previously-defined concepts. It is often motivated informally, usually by an appeal to Intuition (knowledge), intuition or taken to be self-evident. In an axiomatic theory, relations between primitive notions are restricted by axioms. Some authors refer to the latter as "defining" primitive notions by one or more axioms, but this can be misleading. Formal theories cannot dispense with primitive notions, under pain of infinite regress (per the regress problem). For example, in contemporary geometry, ''point (geometry), point'', ''line'', and ''contains'' are some primitive notions. Details Alfred Tarski explained the role of primitive notions as follows: :When we set out to construct a given discipline, we distinguish, first of all, a certain small group of expressions of this discipline that seem to us to be immediately understandable; the expressions in this group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Rankine
William John Macquorn Rankine (; 5 July 1820 ã 24 December 1872) was a Scottish mathematician and physicist. He was a founding contributor, with Rudolf Clausius and William Thomson (Lord Kelvin), to the science of thermodynamics, particularly focusing on its First Law. He developed the Rankine scale, a Fahrenheit-based equivalent to the Celsius-based Kelvin scale of temperature. Rankine developed a complete theory of the steam engine and indeed of all heat engines. His manuals of engineering science and practice were used for many decades after their publication in the 1850s and 1860s. He published several hundred papers and notes on science and engineering topics, from 1840 onwards, and his interests were extremely varied, including, in his youth, botany, music theory and number theory, and, in his mature years, most major branches of science, mathematics and engineering. He was also a singer, pianist and cellist as well as a rifleman. Life Rankine was born in Edinburgh t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Prescott Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist. Joule studied the nature of heat and discovered its relationship to mechanical work. This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI unit of energy, the joule (J), is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the current through a resistor and the heat dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784ã1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in Salford. Joule was tutored as a young man by the famous scientist John Dalton and was strongly in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clifford Truesdell
Clifford Ambrose Truesdell III (February 18, 1919 ã January 14, 2000) was an American mathematician, natural philosopher, and historian of science. Life Truesdell was born in Los Angeles, California. After high school, he spent two years in Europe learning French, German, and Italian, and improving his Latin and Greek. His linguistic skills stood him in good stead in his later historical investigations. At Caltech he was deeply influenced by the teaching of Harry Bateman. In particular, a course in partial differential equations "taught me the difference between an ordinary good teacher and a great mathematician, and after that I never cared what grade I got in anything." He obtained a B.Sc. in mathematics and physics in 1941, and an MSc. in mathematics in 1942. In 1943, he completed a Ph.D. in mathematics at Princeton University. For the rest of the decade, the U.S. Navy employed him to do mechanics research. Truesdell taught at Indiana University 1950ã61, where his students ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Julius Robert Von Mayer
Julius Robert von Mayer (25 November 1814 ã 20 March 1878) was a German physician, chemist, and physicist and one of the founders of thermodynamics. He is best known for enunciating in 1841 one of the original statements of the conservation of energy or what is now known as one of the first versions of the first law of thermodynamics, namely that "energy can be neither created nor destroyed". In 1842, Mayer described the vital chemical process now referred to as oxidation as the primary source of energy for any living creature. He also proposed that plants convert light into chemical energy. His achievements were overlooked and priority for the discovery in 1842 of the ''mechanical equivalent of heat'' was attributed to James Joule in the following year. Early life Mayer was born on 25 November 1814 in Heilbronn, Wû¥rttemberg (Baden-Wû¥rttemberg, modern day Germany), the son of a pharmacist. He grew up in Heilbronn. After completing his ''Abitur'', he studied medicine at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germain Hess
Germain Henri Hess (; 7 August 1802 ã ) was a Swiss-Russian chemist and doctor who formulated Hess' law, an early principle of thermochemistry. Early life and education Hess was born on 7 August 1802 in Geneva, France (Switzerland after 1815). His father was an artist and in 1805 moved the family to the Russian Empire to work as a tutor to a rich family. His mother was a tutor as well and Hess learned German and French at home. In 1817, his family moved to Dorpat, Russian Empire (now Tartu, Estonia), where he went to a private school for two years, and then to Dorpat Gymnasium, which he finished in 1822. In autumn of the same year Hess studied medicine at the University of Dorpat. During that time, the chemistry department was responsible for the Chemistry courses of the Medicine and Pharmacy departments and Professor Gottfried W. Osann was giving the lectures in German (an obvious advance for Hess). Under Osann's supervision, Hess made chemical analyses, but also had an inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
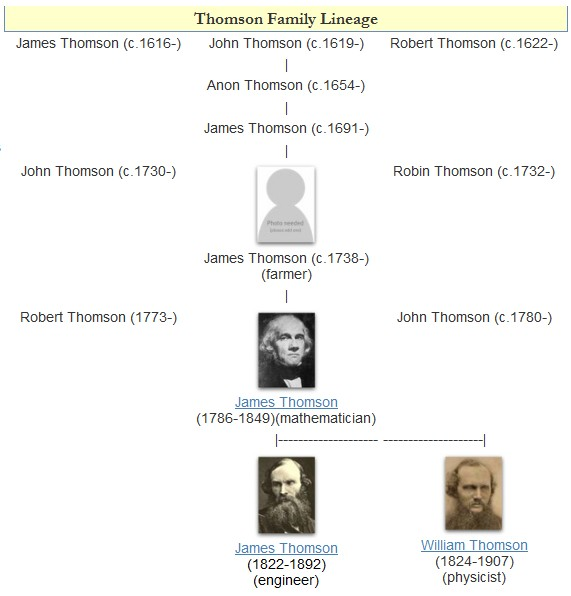
Lord Kelvin
William Thomson, 1st Baron Kelvin (26 June 182417 December 1907), was a British mathematician, Mathematical physics, mathematical physicist and engineer. Born in Belfast, he was the Professor of Natural Philosophy (Glasgow), professor of Natural Philosophy at the University of Glasgow for 53 years, where he undertook significant research on the mathematical analysis of electricity, was instrumental in the formulation of the first and second laws of thermodynamics, and contributed significantly to unifying physics, which was then in its infancy of development as an emerging academic discipline. He received the Royal Society's Copley Medal in 1883 and served as its President of the Royal Society, president from 1890 to 1895. In 1892, he became the first scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in Lord Kelvin's honour. While the existence of a coldest possible temperature, absolute zero, was known before his work, Kelvin d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (physics)
In science, work is the energy transferred to or from an Physical object, object via the application of force along a Displacement (vector), displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the Product (mathematics), product of the force strength and the distance traveled. A force is said to do ''positive work'' if it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by the gravitational force is negative, and is equal to the weight multiplied by the dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |