Palladium on:
[Wikipedia]
[Google]
[Amazon]
Palladium is a
File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Structure of ''α''-PdCl2
File:Pd6Cl12-from-xtal-1996-CM-3D-ellipsoids.png,


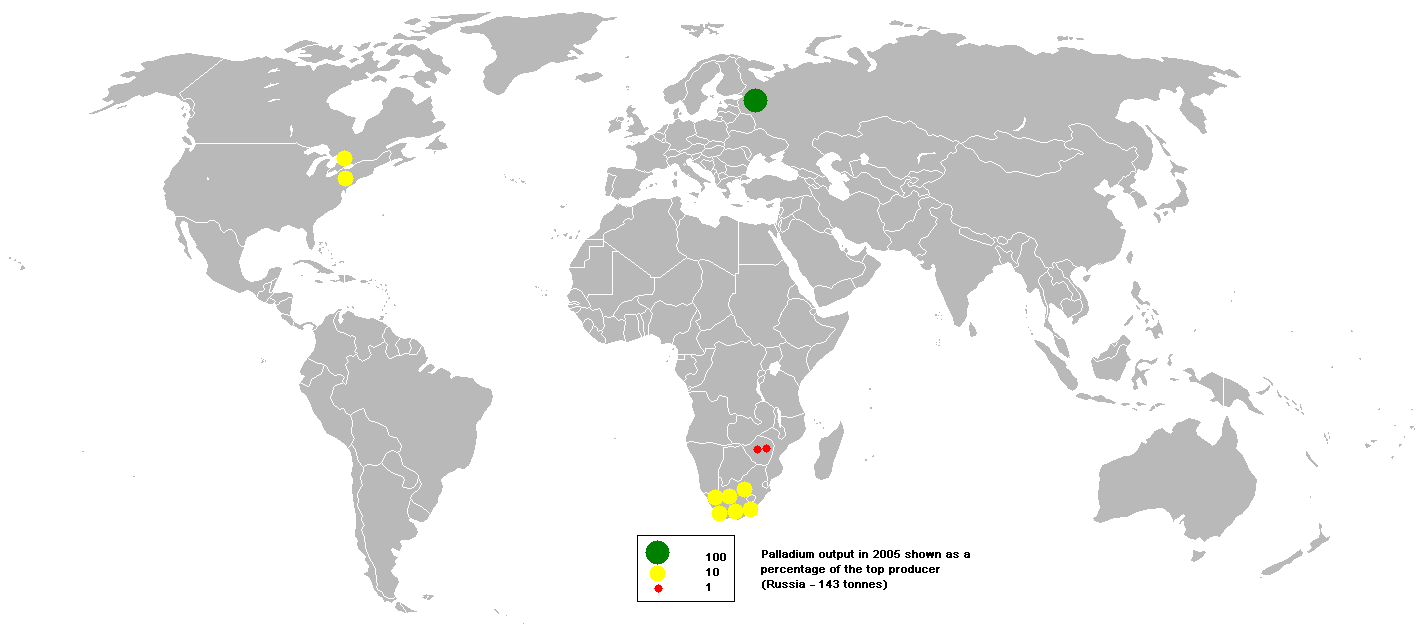 As overall mine production of palladium reached 208,000 kilograms in 2016,
As overall mine production of palladium reached 208,000 kilograms in 2016,

 The largest use of palladium today is in catalytic converters. Palladium is also used in jewelry,
The largest use of palladium today is in catalytic converters. Palladium is also used in jewelry,
 Palladium catalysis is primarily employed in organic chemistry and industrial applications, although its use is growing as a tool for
Palladium catalysis is primarily employed in organic chemistry and industrial applications, although its use is growing as a tool for
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Pd and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston
William Hyde Wollaston (; 6 August 1766 – 22 December 1828) was an English chemist and physicist who is famous for discovering the chemical elements palladium and rhodium. He also developed a way to process platinum ore into malleable ingot ...
. He named it after the asteroid Pallas, which was itself named after the epithet
An epithet (, ), also byname, is a descriptive term (word or phrase) known for accompanying or occurring in place of a name and having entered common usage. It has various shades of meaning when applied to seemingly real or fictitious people, di ...
of the Greek goddess Athena
Athena or Athene, often given the epithet Pallas, is an ancient Greek goddess associated with wisdom, warfare, and handicraft who was later syncretized with the Roman goddess Minerva. Athena was regarded as the patron and protectress of ...
, acquired by her when she slew Pallas
Pallas may refer to:
Astronomy
* 2 Pallas asteroid
** Pallas family, a group of asteroids that includes 2 Pallas
* Pallas (crater), a crater on Earth's moon
Mythology
* Pallas (Giant), a son of Uranus and Gaia, killed and flayed by Athena
* Pa ...
. Palladium, platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
, rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
, ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
, iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
and osmium form a group of elements referred to as the platinum group
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered t ...
metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
More than half the supply of palladium and its congener platinum is used in catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
s, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
, and nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the producti ...
) into nontoxic substances (nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous p ...
). Palladium is also used in electronics, dentistry
Dentistry, also known as dental medicine and oral medicine, is the branch of medicine focused on the teeth, gums, and mouth. It consists of the study, diagnosis, prevention, management, and treatment of diseases, disorders, and conditions o ...
, medicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pract ...
, hydrogen purification A hydrogen purifier is a device to purify hydrogen if hydrogen production is done from hydrocarbon sources, the ultra-high purified hydrogen is needed for applications like PEM fuel cells .
Palladium membrane hydrogen purifiers
The palladium membr ...
, chemical applications, groundwater treatment, and jewelry. Palladium is a key component of fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
s, in which hydrogen and oxygen react to produce electricity, heat, and water.
Ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
deposits
A deposit account is a bank account maintained by a financial institution in which a customer can deposit and withdraw money. Deposit accounts can be savings accounts, current accounts or any of several other types of accounts explained below.
...
of palladium and other PGMs are rare. The most extensive deposits have been found in the norite
Norite is a mafic intrusive igneous rock composed largely of the calcium-rich plagioclase labradorite, orthopyroxene, and olivine. The name ''norite'' is derived from ''Norge'', the Norwegian name for Norway.
Norite also known as orthopyrox ...
belt of the Bushveld Igneous Complex
The Bushveld Igneous Complex (BIC) is the largest layered igneous intrusion within the Earth's crust. It has been tilted and eroded forming the outcrops around what appears to be the edge of a great geological basin: the Transvaal Basin. It i ...
covering the Transvaal Basin
The Transvaal Basin is one of three basins of the Transvaal Supergroup on the Kaapvaal craton. The evolution of this 2.65–2.05 Ga Neoarchaean– Palaeoproterozoic basin is thought to have been derived largely from magmatism, palaeoclimate and ...
in South Africa, the Stillwater Complex in Montana
Montana () is a state in the Mountain West division of the Western United States. It is bordered by Idaho to the west, North Dakota and South Dakota to the east, Wyoming to the south, and the Canadian provinces of Alberta, British Columbi ...
, United States; the Sudbury Basin and Thunder Bay District
Thunder Bay District is a district and census division in Northwestern Ontario in the Canadian province of Ontario. The district seat is Thunder Bay.
In 2016, the population was 146,048. The land area is ; the population density was . Most ...
of Ontario
Ontario ( ; ) is one of the thirteen provinces and territories of Canada.Ontario is located in the geographic eastern half of Canada, but it has historically and politically been considered to be part of Central Canada. Located in Central Ca ...
, Canada, and the Norilsk Complex in Russia. Recycling
Recycling is the process of converting waste materials into new materials and objects. The recovery of energy from waste materials is often included in this concept. The recyclability of a material depends on its ability to reacquire the p ...
is also a source, mostly from scrapped catalytic converters. The numerous applications and limited supply sources result in considerable investment
Investment is the dedication of money to purchase of an asset to attain an increase in value over a period of time. Investment requires a sacrifice of some present asset, such as time, money, or effort.
In finance, the purpose of investing i ...
interest.
Characteristics
Palladium belongs to group 10 in the periodic table, but the configuration in the outermost electrons is in accordance withHund's rule
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration ...
. Electrons that by the Madelung rule
The aufbau principle , from the German ''Aufbauprinzip'' (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells ...
would be expected to occupy the 5 ''s'' instead fill the 4 ''d'' orbitals, as it is more energetically favorable to have a completely filled 4d10 shell instead of the 5s2 4d8 configuration.
This 5s0 configuration, unique in period 5, makes palladium the heaviest element having only ''one'' incomplete electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or ...
, with all shells above it empty.
Palladium has the appearance of a soft silver-white metal that resembles platinum. It is the least dense and has the lowest melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
of the platinum group metals. It is soft and ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
when annealed and is greatly increased in strength and hardness when cold-worked. Palladium dissolves slowly in concentrated nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, in hot, concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
, and when finely ground, in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
. It dissolves readily at room temperature in aqua regia.
Palladium does not react with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
at standard temperature (and thus does not tarnish in air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
). Palladium heated to 800 °C will produce a layer of palladium(II) oxide (PdO). It may slowly develop a slight brownish coloration over time, likely due to the formation of a surface layer of its monoxide.
Palladium films with defects produced by alpha particle bombardment at low temperature exhibit superconductivity having ''T''c=3.2 K.
Isotopes
Naturally occurring palladium is composed of sevenisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s, six of which are stable. The most stable radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s are 107Pd with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 6.5 million years (found in nature), 103Pd with 17 days, and 100Pd with 3.63 days. Eighteen other radioisotopes have been characterized with atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
s ranging from 90.94948(64) u (91Pd) to 122.93426(64) u (123Pd). These have half-lives of less than thirty minutes, except 101Pd (half-life: 8.47 hours), 109Pd (half-life: 13.7 hours), and 112Pd (half-life: 21 hours).
For isotopes with atomic mass unit values less than that of the most abundant stable isotope, 106Pd, the primary decay mode is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
with the primary decay product being rhodium. The primary mode of decay for those isotopes of Pd with atomic mass greater than 106 is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
with the primary product of this decay being silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
.
Radiogenic
A radiogenic nuclide is a nuclide that is produced by a process of radioactive decay. It may itself be radioactive (a radionuclide) or stable (a stable nuclide).
Radiogenic nuclides (more commonly referred to as radiogenic isotopes) form some ...
107Ag is a decay product of 107Pd and was first discovered in 1978 in the Santa Clara meteorite of 1976. The discoverers suggest that the coalescence and differentiation of iron-cored small planets may have occurred 10 million years after a nucleosynthetic
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
event. 107Pd versus Ag correlations observed in bodies, which have been melted since accretion of the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar S ...
, must reflect the presence of short-lived nuclides in the early Solar System. is also produced as a fission product in spontaneous or induced fission of . As it is not very mobile in the environment and has a relatively low decay energy
The decay energy is the energy change of a nucleus having undergone a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation. This decay, or loss of energy ...
, is usually considered to be among the less concerning of the long-lived fission products
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man ...
.
Compounds
Palladium compounds exist primarily in the 0 and +2 oxidation state. Other less common states are also recognized. Generally the compounds of palladium are more similar to those of platinum than those of any other element.Palladium(II)
Palladium(II) chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
is the principal starting material for other palladium compounds. It arises by the reaction of palladium with chlorine. It is used to prepare heterogeneous palladium catalysts such as palladium on barium sulfate, palladium on carbon, and palladium chloride on carbon. Solutions of PdCl2 in nitric acid react with acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
to give palladium(II) acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in ma ...
, also a versatile reagent. PdCl2 reacts with ligands (L) to give square planar complexes of the type PdCl2L2. One example of such complexes is the benzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.
Production
It is p ...
derivative PdX2(PhCN)2.
: PdCl2 + 2 L → PdCl2L2 (L = PhCN, PPh3, NH3, etc)
The complex bis(triphenylphosphine)palladium(II) dichloride
Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupl ...
is a useful catalyst.

Palladium(0)
Palladium forms a range of zerovalent complexes with the formula PdL4, PdL3 and PdL2. For example, reduction of a mixture of PdCl2(PPh3)2 and PPh3 givestetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon de ...
:
:2 PdCl2(PPh3)2 + 4 PPh3 + 5 N2H4 → 2 Pd(PPh3)4 + N2 + 4 N2H5+Cl−
Another major palladium(0) complex, tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or d2(dba)3is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Becau ...
(Pd2(dba)3), is prepared by reducing sodium tetrachloropalladate
Sodium tetrachloropalladate is an inorganic compound with the chemical formula Na2PdCl4. This salt, and the analogous alkali metal salts of the form M2PdCl4, may be prepared simply by reacting palladium(II) chloride with the appropriate alkali meta ...
in the presence of dibenzylideneacetone
Dibenzylideneacetone or dibenzalacetone, often abbreviated dba, is an organic compound with the formula C17H14O. It is a pale-yellow solid insoluble in water, but soluble in ethanol.
It was first prepared in 1881 by the German chemist Rainer Ludwi ...
.
Palladium(0), as well as palladium(II), are catalysts in coupling reactions A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
, as has been recognized by the 2010 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
to Richard F. Heck, Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
, and Akira Suzuki. Such reactions are widely practiced for the synthesis of fine chemicals. Prominent coupling reactions include the Heck, Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
, Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or v ...
, Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electro ...
s, and the Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
. Palladium(II) acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in ma ...
, tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon de ...
(Pd(PPh3)4, and tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0) or d2(dba)3is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Becau ...
(Pd2(dba)3) serve either as catalysts or precatalysts.
Other oxidation states
Although Pd(IV) compounds are comparatively rare, one example is sodium hexachloropalladate(IV), Na2 dCl6 A few compounds of palladium(III) are also known. Palladium(VI) was claimed in 2002, but subsequently disproven. Mixed valence palladium complexes exist, e.g. Pd4(CO)4(OAc)4Pd(acac)2 forms an infinite Pd chain structure, with alternatively interconnected Pd4(CO)4(OAc)4 and Pd(acac)2 units. When alloyed with a moreelectropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
element, palladium can acquire a negative charge. Such compounds are known as palladides, such as gallium palladide
Gallium palladide (GaPd or PdGa) is an intermetallic combination of gallium and palladium. In the Iron monosilicide crystal structure. The compound has been suggested as an improved Hydrogenation#Catalysts, catalyst for hydrogenation reactions. In ...
. Palladides with the stoichiometry RPd3 exist where R is scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
, or any of the lanthanides
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and ytt ...
.
Occurrence
Russia
Russia (, , ), or the Russian Federation, is a List of transcontinental countries, transcontinental country spanning Eastern Europe and North Asia, Northern Asia. It is the List of countries and dependencies by area, largest country in the ...
was the top producer with 82,000 kilograms, followed by South Africa, Canada and the U.S. Russia's company Norilsk Nickel
Norilsk Nickel (russian: ГМК «Норильский никель»), or Nornickel, is a Russian nickel and palladium mining and smelting company. Its largest operations are located in the Norilsk–Talnakh area near the Yenisei River in the no ...
ranks first among the largest palladium producers globally, accounting for 39% of the world's production.
Palladium can be found as a free metal alloyed with gold and other platinum-group metals in placer deposits of the Ural Mountains
The Ural Mountains ( ; rus, Ура́льские го́ры, r=Uralskiye gory, p=ʊˈralʲskʲɪjə ˈɡorɨ; ba, Урал тауҙары) or simply the Urals, are a mountain range that runs approximately from north to south through western ...
, Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
, Ethiopia
Ethiopia, , om, Itiyoophiyaa, so, Itoobiya, ti, ኢትዮጵያ, Ítiyop'iya, aa, Itiyoppiya officially the Federal Democratic Republic of Ethiopia, is a landlocked country in the Horn of Africa. It shares borders with Eritrea to the ...
, North
North is one of the four compass points or cardinal directions. It is the opposite of south and is perpendicular to east and west. ''North'' is a noun, adjective, or adverb indicating Direction (geometry), direction or geography.
Etymology
T ...
and South America
South America is a continent entirely in the Western Hemisphere and mostly in the Southern Hemisphere, with a relatively small portion in the Northern Hemisphere at the northern tip of the continent. It can also be described as the southe ...
. For the production of palladium, these deposits play only a minor role. The most important commercial sources are nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
-copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
deposits found in the Sudbury Basin, Ontario
Ontario ( ; ) is one of the thirteen provinces and territories of Canada.Ontario is located in the geographic eastern half of Canada, but it has historically and politically been considered to be part of Central Canada. Located in Central Ca ...
, and the Norilsk–Talnakh deposits in Siberia
Siberia ( ; rus, Сибирь, r=Sibir', p=sʲɪˈbʲirʲ, a=Ru-Сибирь.ogg) is an extensive geographical region, constituting all of North Asia, from the Ural Mountains in the west to the Pacific Ocean in the east. It has been a part of ...
. The other large deposit is the Merensky Reef
The Merensky Reef is a layer of igneous rock in the Bushveld Igneous Complex (BIC) in the North West, Limpopo, Gauteng and Mpumalanga provinces of South Africa which together with an underlying layer, the Upper Group 2 Reef (UG2), contains most o ...
platinum group
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered t ...
metals deposit within the Bushveld Igneous Complex
The Bushveld Igneous Complex (BIC) is the largest layered igneous intrusion within the Earth's crust. It has been tilted and eroded forming the outcrops around what appears to be the edge of a great geological basin: the Transvaal Basin. It i ...
South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countri ...
. The Stillwater igneous complex
The Stillwater igneous complex is a large Layered intrusion, layered mafic intrusion (LMI) located in southern Montana in Stillwater County, Montana, Stillwater, Sweet Grass County, Montana, Sweet Grass and Park County, Montana, Park Counties. ...
of Montana
Montana () is a state in the Mountain West division of the Western United States. It is bordered by Idaho to the west, North Dakota and South Dakota to the east, Wyoming to the south, and the Canadian provinces of Alberta, British Columbi ...
and the Roby zone ore body of the Lac des Îles igneous complex
The Lac des Îles igneous complex of northwestern Ontario, Canada is a layered gabbroic intrusion which is the host for the largest palladium orebody in Canada. The orebody is currently being mined as a combined open pit and underground operation ...
of Ontario are the two other sources of palladium in Canada and the United States. Palladium is found in the rare minerals cooperite and polarite
Polarite, is an opaque, yellow-white mineral with the chemical formula . Its crystals are orthorhombic pyramidal, but can only be seen through a microscope. It has a metallic luster and leaves a white streak. Polarite is rated 3.5 to 4 on the M ...
. Many more Pd minerals are known, but all of them are very rare.
Palladium is also produced in nuclear fission reactors and can be extracted from spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
(see synthesis of precious metals), though this source for palladium is not used. None of the existing nuclear reprocessing facilities are equipped to extract palladium from the high-level radioactive waste
High-level waste (HLW) is a type of nuclear waste created by the reprocessing of spent nuclear fuel. It exists in two main forms:
* First and second cycle raffinate and other waste streams created by nuclear reprocessing.
* Waste formed by vit ...
. A complication for the recovery of Palladium in spent fuel is the presence of a slightly radioactive long-lived fission product. Depending on end use, the radioactivity contributed by the might make the recovered Palladium unusable without a costly step of isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" n ...
.
Applications

 The largest use of palladium today is in catalytic converters. Palladium is also used in jewelry,
The largest use of palladium today is in catalytic converters. Palladium is also used in jewelry, dentistry
Dentistry, also known as dental medicine and oral medicine, is the branch of medicine focused on the teeth, gums, and mouth. It consists of the study, diagnosis, prevention, management, and treatment of diseases, disorders, and conditions o ...
, watch
A watch is a portable timepiece intended to be carried or worn by a person. It is designed to keep a consistent movement despite the motions caused by the person's activities. A wristwatch is designed to be worn around the wrist, attached by ...
making, blood sugar test strips, aircraft spark plugs, surgical instrument
A surgical instrument is a tool or device for performing specific actions or carrying out desired effects during a surgery or operation, such as modifying biological tissue, or to provide access for viewing it. Over time, many different kinds of ...
s, and electrical contact
An electrical contact is an electrical circuit component found in electrical switches, relays, connectors and circuit breakers. Each contact is a piece of electrically conductive material, typically metal. When a pair of contacts touch, they c ...
s. Palladium is also used to make professional transverse (concert or classical) flutes. As a commodity, palladium bullion
Bullion is non-ferrous metal that has been refined to a high standard of elemental purity. The term is ordinarily applied to bulk metal used in the production of coins and especially to precious metals such as gold and silver. It comes from t ...
has ISO currency code
ISO 4217 is a standard published by the International Organization for Standardization (ISO) that defines alpha codes and numeric codes for the representation of currencies and provides information about the relationships between individual ...
s of XPD and 964. Palladium is one of only four metals to have such codes, the others being gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
, silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
and platinum. Because it adsorbs
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a Surface science, surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorpti ...
hydrogen, palladium was a key component of the controversial cold fusion
Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature. It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and p ...
experiments of the late 1980s.
Catalysis
When it is finely divided, as withpalladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.
Uses Hydrogenation
Palladium on carbon is used for catalytic hydrog ...
, palladium forms a versatile catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
; it speeds heterogeneous catalytic processes like hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
, dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
, and petroleum cracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic compound, organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, b ...
. Palladium is also essential to the Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. w ...
, also called Lindlar's Palladium. A large number of carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed bet ...
ing reactions in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
are facilitated by palladium compound catalysts. For example:
* Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a sub ...
* Suzuki coupling
* Tsuji-Trost reactions
* Wacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
* Negishi reaction
* Stille coupling
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electroph ...
* Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or v ...
(See palladium compounds
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
and palladium-catalyzed coupling reactions
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
.)
When dispersed on conductive materials, palladium is an excellent electrocatalyst for oxidation of primary alcohols in alkaline media. Palladium is also a versatile metal for homogeneous catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysi ...
, used in combination with a broad variety of ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s for highly selective chemical transformations.
In 2010 the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
was awarded "for palladium-catalyzed cross couplings in organic synthesis" to Richard F. Heck, Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
and Akira Suzuki. A 2008 study showed that palladium is an effective catalyst for carbon-fluorine bonds.
 Palladium catalysis is primarily employed in organic chemistry and industrial applications, although its use is growing as a tool for
Palladium catalysis is primarily employed in organic chemistry and industrial applications, although its use is growing as a tool for synthetic biology
Synthetic biology (SynBio) is a multidisciplinary area of research that seeks to create new biological parts, devices, and systems, or to redesign systems that are already found in nature.
It is a branch of science that encompasses a broad ran ...
; in 2017, effective ''in vivo'' catalytic activity of palladium nanoparticles was demonstrated in mammals to treat disease.
Electronics
The primary application of palladium in electronics is in multi-layer ceramic capacitors in which palladium (and palladium-silver alloy) is used for electrodes. Palladium (sometimes alloyed with nickel) is or can be used for component and connector plating in consumer electronics and in soldering materials. The electronic sector consumed of palladium in 2006, according to aJohnson Matthey
Johnson Matthey is a British multinational speciality chemicals and sustainable technologies company headquartered in London, England. It is listed on the London Stock Exchange and is a constituent of the FTSE 250 Index.
History Early years
Jo ...
report.
Technology
Hydrogen easily diffuses through heated palladium, andmembrane reactor
A membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to add reactants or remove products of the reaction.
Chemical reactors making use of membranes are usually referred to as membr ...
s with Pd membranes are used in the production of high purity hydrogen. Palladium is used in palladium-hydrogen electrode The palladium-hydrogen electrode (abbreviation: Pd/H2) is one of the common reference electrodes used in electrochemical study. Most of its characteristics are similar to the standard hydrogen electrode (with platinum). But palladium has one signif ...
s in electrochemical studies. Palladium(II) chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
readily catalyzes carbon monoxide gas to carbon dioxide and is useful in carbon monoxide detector
A carbon monoxide detector or CO detector is a device that detects the presence of the carbon monoxide (CO) gas to prevent carbon monoxide poisoning. In the late 1990s Underwriters Laboratories changed the definition of a single station CO de ...
s.
Hydrogen storage
Palladium readilyadsorbs
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a Surface science, surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorpti ...
hydrogen at room temperatures, forming palladium hydride Palladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At r ...
PdHx with x less than 1. While this property is common to many transition metals, palladium has a uniquely high absorption capacity and does not lose its ductility until x approaches 1. This property has been investigated in designing an efficient, inexpensive, and safe hydrogen fuel storage medium, though palladium itself is currently prohibitively expensive for this purpose. The content of hydrogen in palladium can be linked to magnetic susceptibility, which decreases with the increase of hydrogen and becomes zero for PdH0.62. At any higher ratio, the solid solution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The wor ...
becomes diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
.
Palladium is also used for hydrogen purification via hydrogen-purification membranes.
Dentistry
Palladium is used in small amounts (about 0.5%) in some alloys ofdental amalgam
Dental amalgam is a liquid mercury and metal alloy mixture used in dentistry to fill cavities caused by tooth decay. Low-copper amalgam commonly consists of mercury (50%), silver (~22–32%), tin (~14%), zinc (~8%) and other trace metals.
...
to decrease corrosion and increase the metallic lustre of the final restoration.
Jewelry
Palladium has been used as a precious metal in jewelry since 1939 as an alternative to platinum in the alloys called "white gold
Pure gold is slightly reddish yellow in color, but colored gold in various other colors can be produced by alloying gold with other elements.
Colored golds can be classified in three groups:
* Alloys with silver and copper in various proportions ...
", where the naturally white color of palladium does not require rhodium plating. Palladium is much less dense than platinum. Similar to gold, palladium can be beaten into leaf
A leaf ( : leaves) is any of the principal appendages of a vascular plant stem, usually borne laterally aboveground and specialized for photosynthesis. Leaves are collectively called foliage, as in "autumn foliage", while the leaves, ste ...
as thin as 100 nm ( in). Unlike platinum, palladium may discolor at temperatures above due to oxidation, making it more brittle and thus less suitable for use in jewelry; to prevent this, palladium intended for jewelry is heated under controlled conditions.
Prior to 2004, the principal use of palladium in jewelry was the manufacture of white gold. Palladium is one of the three most popular alloying metals in white gold (nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
and silver can also be used). Palladium-gold is more expensive than nickel-gold, but seldom causes allergic reactions (though certain cross-allergies with nickel may occur).
When platinum became a strategic resource during World War II, many jewelry bands were made out of palladium. Palladium was little used in jewelry because of the technical difficulty of casting
Casting is a manufacturing process in which a liquid material is usually poured into a mold, which contains a hollow cavity of the desired shape, and then allowed to solidify. The solidified part is also known as a ''casting'', which is ejected ...
. With the casting problem resolved the use of palladium in jewelry increased, originally because platinum increased in price while the price of palladium decreased. In early 2004, when gold and platinum prices rose steeply, China began fabricating volumes of palladium jewelry, consuming 37 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s in 2005. Subsequent changes in the relative price of platinum lowered demand for palladium to 17.4 tonnes in 2009. Demand for palladium as a catalyst has increased the price of palladium to about 50% higher than that of platinum in January 2019.
In January 2010, hallmark
A hallmark is an official mark or series of marks struck on items made of metal, mostly to certify the content of noble metals—such as platinum, gold, silver and in some nations, palladium. In a more general sense, the term '' hallmark'' can a ...
s for palladium were introduced by assay offices in the United Kingdom, and hallmarking became mandatory for all jewelry advertising pure or alloyed palladium. Articles can be marked as 500, 950, or 999 parts of palladium per thousand of the alloy.
Fountain pen
A fountain pen is a writing instrument which uses a metal nib to apply a water-based ink to paper. It is distinguished from earlier dip pens by using an internal reservoir to hold ink, eliminating the need to repeatedly dip the pen in an in ...
nibs made from gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
are sometimes plated with palladium when a silver (rather than gold) appearance is desired. Sheaffer
Sheaffer Pen Corporation () is an American manufacturing company of writing instruments, particularly luxury fountain pens. The company was founded by Walter A. Sheaffer in Fort Madison, Iowa, and incorporated in 1913 to exploit his invention of ...
has used palladium plating for decades, either as an accent on otherwise gold nibs or covering the gold completely.
Palladium is also used by the luxury brand Hermes as one of the metals plating the hardware on their handbags, most famous of which being the highly sought after Birkin.
Photography
In theplatinotype
Platinum prints, also called ''platinotypes'', are photographic prints made by a monochrome printing process involving platinum.
Platinum tones range from warm black, to reddish brown, to expanded mid-tone grays that are unobtainable in silver ...
printing process, photographers make fine-art black-and-white prints using platinum or palladium salts. Often used with platinum, palladium provides an alternative to silver.
Effects on health
Toxicity
Palladium is a metal with low toxicity as conventionally measured (e.g. LD50). Recent research on the mechanism of palladium toxicity suggests high toxicity if measured on a longer timeframe and at the cellular level in the liver and kidney. Mitochondria appear to have a key role in palladium toxicity via mitochondrial membrane potential collapse and depletion of the cellular glutathione (GSH) level. Until that recent work, it had been thought that palladium was poorly absorbed by the human body wheningested
Ingestion is the consumption of a substance by an organism. In animals, it normally is accomplished by taking in a substance through the mouth into the gastrointestinal tract, such as through eating or drinking. In single-celled organisms inges ...
. Plants such as the water hyacinth
''Pontederia crassipes'' (formerly ''Eichhornia crassipes''), commonly known as common water hyacinth is an aquatic plant native to South America, naturalized throughout the world, and often invasive outside its native range.rodents suggest it may be
 William Hyde Wollaston noted the
William Hyde Wollaston noted the
 Global palladium sales were 8.84 million ounces (250.6 tonnes) in 2017, of which 86% was used in the manufacturing of automotive catalytic converters, followed by industrial, jewelry, and investment usages. More than 75% of global platinum and 40% of palladium are mined in
Global palladium sales were 8.84 million ounces (250.6 tonnes) in 2017, of which 86% was used in the manufacturing of automotive catalytic converters, followed by industrial, jewelry, and investment usages. More than 75% of global platinum and 40% of palladium are mined in
Palladium
at ''
Current and Historical Palladium Price
* {{Authority control Chemical elements Noble metals Transition metals Precious metals Native element minerals Chemical elements with face-centered cubic structure
carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subs ...
, though until the recent research cited above, no clear evidence indicated that the element harms humans.
Precautions
Like other platinum-group metals, bulk Pd is quite inert. Althoughcontact dermatitis
Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are ...
has been reported, data on the effects are limited. It has been shown that people with an allergic reaction to palladium also react to nickel, making it advisable to avoid the use of dental alloys containing palladium on those so allergic.
Some palladium is emitted with the exhaust gases of cars with catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
s. Between 4 and 108 ng/km of palladium particulate is released by such cars, while the total uptake from food is estimated to be less than 2 µg per person a day. The second possible source of palladium is dental restoration, from which the uptake of palladium is estimated to be less than 15 µg per person per day. People working with palladium or its compounds might have a considerably greater uptake. For soluble compounds such as palladium chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
, 99% is eliminated from the body within 3 days.
The median lethal dose (LD50) of soluble palladium compounds in mice is 200 mg/kg for oral
The word oral may refer to:
Relating to the mouth
* Relating to the mouth, the first portion of the alimentary canal that primarily receives food and liquid
**Oral administration of medicines
** Oral examination (also known as an oral exam or or ...
and 5 mg/kg for intravenous administration
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutri ...
.
History
 William Hyde Wollaston noted the
William Hyde Wollaston noted the discovery
Discovery may refer to:
* Discovery (observation), observing or finding something unknown
* Discovery (fiction), a character's learning something unknown
* Discovery (law), a process in courts of law relating to evidence
Discovery, The Discover ...
of a new noble metal in July 1802 in his lab book and named it palladium in August of the same year. Wollaston purified a quantity of the material and offered it, without naming the discoverer, in a small shop in Soho
Soho is an area of the City of Westminster, part of the West End of London. Originally a fashionable district for the aristocracy, it has been one of the main entertainment districts in the capital since the 19th century.
The area was develo ...
in April 1803. After harsh criticism from Richard Chenevix, who claimed that palladium was an alloy of platinum and mercury, Wollaston anonymously offered a reward of £20 for 20 grains of synthetic palladium ''alloy''. Chenevix received the Copley Medal
The Copley Medal is an award given by the Royal Society, for "outstanding achievements in research in any branch of science". It alternates between the physical sciences or mathematics and the biological sciences. Given every year, the medal is t ...
in 1803 after he published his experiments on palladium. Wollaston published the discovery of rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
in 1804 and mentions some of his work on palladium. He disclosed that he was the discoverer of palladium in a publication in 1805.
It was named by Wollaston in 1802 after the asteroid 2 Pallas, which had been discovered two months earlier. Wollaston found palladium in crude platinum ore from South America
South America is a continent entirely in the Western Hemisphere and mostly in the Southern Hemisphere, with a relatively small portion in the Northern Hemisphere at the northern tip of the continent. It can also be described as the southe ...
by dissolving the ore in aqua regia, neutralizing the solution with sodium hydroxide, and precipitating platinum as ammonium chloroplatinate
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2 tCl6 It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. ...
with ammonium chloride. He added mercuric cyanide
Mercury(II) cyanide, also known as mercuric cyanide, is a compound of mercury. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia; slightly soluble in ether; and insoluble in benzene ...
to form the compound palladium(II) cyanide
Palladium(II) dicyanide is the inorganic compound with the formula Pd(CN)2. A grey solid, it is a coordination polymer. It was the first palladium compound isolated in pure form. In his attempts to produce pure platinum metal in 1804, W.H. Wolla ...
, which was heated to extract palladium metal.
Palladium chloride
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value ...
was at one time prescribed as a tuberculosis
Tuberculosis (TB) is an infectious disease usually caused by '' Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in ...
treatment at the rate of 0.065 g per day (approximately one milligram per kilogram of body weight). This treatment had many negative side-effects
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequence ...
, and was later replaced by more effective drugs.
Most palladium is used for catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
s in the automobile industry. Catalytic converters are targets for thieves because they contain palladium and other rare metals. In the run up to year 2000, the Russian supply of palladium to the global market was repeatedly delayed and disrupted; for political reasons, the export quota was not granted on time. The ensuing market panic drove the price to an all-time high of in January 2001. Around that time, the Ford Motor Company
Ford Motor Company (commonly known as Ford) is an American multinational automobile manufacturer headquartered in Dearborn, Michigan, United States. It was founded by Henry Ford and incorporated on June 16, 1903. The company sells automobi ...
, fearing that automobile production would be disrupted by a palladium shortage, stockpiled the metal. When prices fell in early 2001, Ford lost nearly US$
The United States dollar (symbol: $; code: USD; also abbreviated US$ or U.S. Dollar, to distinguish it from other dollar-denominated currencies; referred to as the dollar, U.S. dollar, American dollar, or colloquially buck) is the official ...
1 billion.
World demand for palladium increased from 100 tons in 1990 to nearly 300 tons in 2000. The global production of palladium from mines was 222 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s in 2006 according to the United States Geological Survey
The United States Geological Survey (USGS), formerly simply known as the Geological Survey, is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, ...
. Many were concerned about a steady supply of palladium in the wake of Russia's annexation of Crimea, partly as sanctions could hamper Russian palladium exports; any restrictions on Russian palladium exports could have exacerbated what was already expected to be a large palladium deficit in 2014. Those concerns pushed palladium prices to their highest level since 2001. In September 2014 they soared above the $900 per ounce mark. In 2016 however palladium cost around $614 per ounce as Russia managed to maintain stable supplies. In January 2019 palladium futures
Futures may mean:
Finance
*Futures contract, a tradable financial derivatives contract
*Futures exchange, a financial market where futures contracts are traded
* ''Futures'' (magazine), an American finance magazine
Music
* ''Futures'' (album), a ...
climbed past $1,344 per ounce for the first time on record, mainly due to the strong demand from the automotive industry. Palladium reached on 6 January 2020, passing $2,000 per troy ounce the first time. The price rose above $3,000 per troy ounce in May 2021 and March 2022.
Palladium as investment
 Global palladium sales were 8.84 million ounces (250.6 tonnes) in 2017, of which 86% was used in the manufacturing of automotive catalytic converters, followed by industrial, jewelry, and investment usages. More than 75% of global platinum and 40% of palladium are mined in
Global palladium sales were 8.84 million ounces (250.6 tonnes) in 2017, of which 86% was used in the manufacturing of automotive catalytic converters, followed by industrial, jewelry, and investment usages. More than 75% of global platinum and 40% of palladium are mined in South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countri ...
. Russia's mining company, Norilsk Nickel
Norilsk Nickel (russian: ГМК «Норильский никель»), or Nornickel, is a Russian nickel and palladium mining and smelting company. Its largest operations are located in the Norilsk–Talnakh area near the Yenisei River in the no ...
, produces another 44% of palladium, with US and Canada-based mines producing most of the rest.
The price for palladium reached an all-time high of $2,981.40 per ounce on May 3, 2021 driven mainly on speculation of the catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
demand from the automobile industry
The automotive industry comprises a wide range of companies and organizations involved in the design, development, manufacturing, marketing, and selling of motor vehicles. It is one of the world's largest industries by revenue (from 16 % such ...
. Palladium is traded in the spot market
The spot market or cash market is a public financial market in which financial instruments or commodities are traded for immediate delivery. It contrasts with a futures market, in which delivery is due at a later date. In a spot market, settle ...
with the code "XPD". When settled in USD, the code is "XPDUSD". A later surplus of the metal was caused by the Russian government selling stockpiles from the Soviet Era
The history of Soviet Russia and the Soviet Union (USSR) reflects a period of change for both Russia and the world. Though the terms "Soviet Russia" and "Soviet Union" often are synonymous in everyday speech (either acknowledging the dominance ...
, at a rate of about 1.6 to 2 million ounces (45.4 to 56.7 t) a year. The amount and status of this stockpile are a state secret.
During the Russo-Ukrainian War
The Russo-Ukrainian War; uk, російсько-українська війна, rosiisko-ukrainska viina. has been ongoing between Russia (alongside Russian separatist forces in Donbas, Russian separatists in Ukraine) and Ukraine since Feb ...
in March 2022, prices for palladium increased 13%, since the first of March. Russia is the primary supplier to Europe and the country supplies 37% of the global production.
Palladium producers
*Norilsk Nickel
Norilsk Nickel (russian: ГМК «Норильский никель»), or Nornickel, is a Russian nickel and palladium mining and smelting company. Its largest operations are located in the Norilsk–Talnakh area near the Yenisei River in the no ...
(, ), palladium powder and ingots.
* North American Palladium (), Canada's largest producer of palladium operating the Lac des Iles palladium mine near Thunder Bay, Ontario
Thunder Bay is a city in and the seat of Thunder Bay District, Ontario, Canada. It is the most populous municipality in Northwestern Ontario and the second most populous (after Greater Sudbury) municipality in Northern Ontario; its population ...
.
* Stillwater Mining (), a major North American palladium miner in Montana.
Exchange-traded products
WisdomTree Physical Palladium () is backed by allocated palladiumbullion
Bullion is non-ferrous metal that has been refined to a high standard of elemental purity. The term is ordinarily applied to bulk metal used in the production of coins and especially to precious metals such as gold and silver. It comes from t ...
and was the world's first palladium ETF. It is listed on the London Stock Exchange
London Stock Exchange (LSE) is a stock exchange in the City of London, England, United Kingdom. , the total market value of all companies trading on LSE was £3.9 trillion. Its current premises are situated in Paternoster Square close to St P ...
as PHPD, Xetra Trading System, Euronext
Euronext N.V. (short for European New Exchange Technology) is a pan-European bourse that offers various trading and post-trade services.
Traded assets include regulated equities, exchange-traded funds (ETF), warrants and certificates, bonds, ...
and Milan
Milan ( , , Lombard: ; it, Milano ) is a city in northern Italy, capital of Lombardy, and the second-most populous city proper in Italy after Rome. The city proper has a population of about 1.4 million, while its metropolitan city h ...
. ETFS Physical Palladium Shares () is an ETF traded on the New York Stock Exchange
The New York Stock Exchange (NYSE, nicknamed "The Big Board") is an American stock exchange in the Financial District of Lower Manhattan in New York City. It is by far the world's largest stock exchange by market capitalization of its listed ...
.
Bullion coins and bars
A traditional way of investing in palladium is buying bullion coins and bars made of palladium. Available palladium coins include theCanadian Palladium Maple Leaf
The Canadian Palladium Maple Leaf is the official bullion palladium coin of Canada. It is issued by the Royal Canadian Mint in .9995 purity. The coins have legal tender status in Canada, but as is often the case with bullion coins, the face val ...
, the Chinese Panda, and the American Palladium Eagle
The American Palladium Eagle is the official palladium bullion coin of the United States. Each coin has a face value of $25 and is composed of 99.95% fine palladium, with 1 troy ounce actual palladium weight.
History
The Palladium Eagle was aut ...
. The liquidity
Liquidity is a concept in economics involving the convertibility of assets and obligations. It can include:
* Market liquidity, the ease with which an asset can be sold
* Accounting liquidity, the ability to meet cash obligations when due
* Liq ...
of direct palladium bullion investment is poorer than that of gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
because there is low circulation of palladium coins.
See also
* 2000s commodities boom * 2020s commodities boom *Bullion
Bullion is non-ferrous metal that has been refined to a high standard of elemental purity. The term is ordinarily applied to bulk metal used in the production of coins and especially to precious metals such as gold and silver. It comes from t ...
* Bullion coin
Bullion is non-ferrous metal that has been refined to a high standard of elemental purity. The term is ordinarily applied to bulk metal used in the production of coins and especially to precious metals such as gold and silver. It comes fro ...
* Inflation hedge An inflation hedge is an investment intended to protect the investor against (hedge) a decrease in the purchasing power of money (inflation). There is no investment known to be a successful hedge in all inflationary environments, just as there is n ...
* Pseudo palladium
Pseudo palladium (RhAg) is a binary alloy consisting of equal parts of rhodium (atomic number 45) and silver (atomic number 47) created using nanotechnology to create a far more homogeneous mixture than might be possible using more conventional ...
References
External links
Palladium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Current and Historical Palladium Price
* {{Authority control Chemical elements Noble metals Transition metals Precious metals Native element minerals Chemical elements with face-centered cubic structure