|
Electron Shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (''n'' = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell. Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the ''n''th shell can in principle hold up to 2( ''n''2) electrons. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Barkla
Charles Glover Barkla FRS FRSE (7 June 1877 – 23 October 1944) was a British physicist, and the winner of the Nobel Prize in Physics in 1917 for his work in X-ray spectroscopy and related areas in the study of X-rays (Roentgen rays). Life Barkla was born in Widnes, England, to John Martin Barkla, a secretary for the Atlas Chemical Company, and Sarah Glover, daughter of a watchmaker. Barkla studied at the Liverpool Institute and proceeded to Liverpool University with a County Council Scholarship and a Bibby Scholarship. Barkla initially studied Mathematics but later specialised in Physics under Sir Oliver Lodge. During the absence of Oliver Lodge due to ill health, Barkla replaced him in lectures. In 1899 Barkla was admitted to Trinity College, Cambridge, with an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851, to work in the Cavendish Laboratory under the physicist J. J. Thomson (discoverer of the electron). During his first two years at Cam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
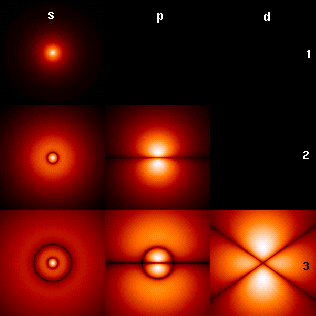
Magnetic Quantum Number
In atomic physics, the magnetic quantum number () is one of the four quantum numbers (the other three being the principal, azimuthal, and spin) which describe the unique quantum state of an electron. The magnetic quantum number distinguishes the orbitals available within a subshell, and is used to calculate the azimuthal component of the orientation of orbital in space. Electrons in a particular subshell (such as s, p, d, or f) are defined by values of (0, 1, 2, or 3). The value of can range from to , including zero. Thus the s, p, d, and f subshells contain 1, 3, 5, and 7 orbitals each, with values of within the ranges 0, ±1, ±2, ±3 respectively. Each of these orbitals can accommodate up to two electrons (with opposite spins), forming the basis of the periodic table. Derivation There is a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, \ell, m_\ell, and s specify the complete quantum state of a single electron in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azimuthal Quantum Number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number, the magnetic quantum number, and the spin quantum number). It is also known as the orbital angular momentum quantum number, orbital quantum number or second quantum number, and is symbolized as ℓ (pronounced ''ell''). Derivation Connected with the energy states of the atom's electrons are four quantum numbers: ''n'', ''ℓ'', ''m''''ℓ'', and ''m''''s''. These specify the complete, unique quantum state of a single electron in an atom, and make up its wavefunction or ''orbital''. When solving to obtain the wave function, the Schrödinger equation reduces to three equations that lead to the first three quantum numbers. Therefore, the equations for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character (keeping their own electrons) increasing from left to right across a period, and from down to up across a group, and metallic character (surrendering electrons to other atoms) increasing in the opposite direction. The underlying reason for these trends is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilbert N
Gilbert may refer to: People and fictional characters *Gilbert (given name), including a list of people and fictional characters *Gilbert (surname), including a list of people Places Australia * Gilbert River (Queensland) * Gilbert River (South Australia) Kiribati * Gilbert Islands, a chain of atolls and islands in the Pacific Ocean United States * Gilbert, Arizona, a town * Gilbert, Arkansas, a town * Gilbert, Florida, the airport of Winterhaven * Gilbert, Iowa, a city * Gilbert, Louisiana, a village * Gilbert, Michigan, and unincorporated community * Gilbert, Minnesota, a city * Gilbert, Nevada, ghost town * Gilbert, Ohio, an unincorporated community * Gilbert, Pennsylvania, an unincorporated community * Gilbert, South Carolina, a town * Gilbert, West Virginia, a town * Gilbert, Wisconsin, an unincorporated community * Mount Gilbert (other), various mountains * Gilbert River (Oregon) Outer space * Gilbert (lunar crater) * Gilbert (Martian crater) Arts and ente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Rugeley Bury
Charles Rugeley Bury (29 June 1890 – 30 December 1968) was an English physical chemist who proposed an early model of the atom with the arrangement of electrons, which explained their chemical properties, alongside the more dominant model of Niels Bohr. In some early papers, the model was called the "Bohr-Bury Atom". He introduced the word ''transition'' to describe the elements now known as transition metals or transition elements. Bury was born in Henley-on-Thames and grew up in Ellfield, Wotton-under-Edge. His father had studied law but did not continue in the field and died when he was young. A grandmother in Leamington took care of him and his early education was at Malvern College. He then went to Trinity College, Oxford where D.H. Nagel was a tutor. His chemistry teachers included Harold Hartley. Bury worked as a demonstrator in Balliol and Trinity College labs. In 1912 he went to Göttingen and worked with Walther Nernst. He then joined the chemistry department at the U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry. Langmuir's most famous publication is the 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his "concentric theory of atomic structure". Langmuir became embroiled in a priority dispute with Lewis over this work; Langmuir's presentation skills were largely responsible for the popularization of the theory, although the credit for the theory itself belongs mostly to Lewis. While at General Electric from 1909 to 1950, Langmuir advanced several fields of physics and chemistry, inventing the gas-filled incandescent lamp and the hydrogen welding technique. The Langmuir Laboratory for Atmospheric Research near Socorro, New Mexico, was named in his honor, as wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory of relativity, but he also made important contributions to the development of the theory of quantum mechanics. Relativity and quantum mechanics are the two pillars of modern physics. His mass–energy equivalence formula , which arises from relativity theory, has been dubbed "the world's most famous equation". His work is also known for its influence on the philosophy of science. He received the 1921 Nobel Prize in Physics "for his services to theoretical physics, and especially for his discovery of the law of the photoelectric effect", a pivotal step in the development of quantum theory. His intellectual achievements and originality resulted in "Einstein" becoming synonymous with "genius". In 1905, a year sometimes described as his ''annu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siegbahn Notation
The Siegbahn notation is used in X-ray spectroscopy to name the spectral lines that are characteristic to elements. It was introduced by Manne Siegbahn. The characteristic lines in X-ray emission spectra correspond to atomic electronic transitions where an electron jumps down to a vacancy in one of the inner shells of an atom. Such a hole in an inner shell may have been produced by bombardment with electrons in an X-ray tube, by other particles as in PIXE, by other X-rays in X-ray fluorescence or by radioactive decay of the atom's nucleus. Although still widely used in spectroscopy, this notation is unsystematic and often confusing. For these reasons, International Union of Pure and Applied Chemistry (IUPAC) recommends another nomenclature. History The use of the letters K and L to denote X-rays originates in a 1911 paper by Charles Glover Barkla, titled ''The Spectra of the Fluorescent Röntgen Radiations'' ("Röntgen radiation" is an archaic name for "X-rays"). By 1913, Henry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |







.jpg)