|
Radiogenic
A radiogenic nuclide is a nuclide that is produced by a process of radioactive decay. It may itself be radioactive (a radionuclide) or stable (a stable nuclide). Radiogenic nuclides (more commonly referred to as radiogenic isotopes) form some of the most important tools in geology. They are used in two principal ways: #In comparison with the quantity of the radioactive 'parent isotope' in a system, the quantity of the radiogenic 'daughter product' is used as a radiometric dating tool (e.g. uranium–lead geochronology). #In comparison with the quantity of a non-radiogenic isotope of the same element, the quantity of the radiogenic isotope is used to define its isotopic signature (e.g. 206Pb/204Pb). This technique is discussed in more detail under the heading isotope geochemistry. Examples Some naturally occurring isotopes are entirely radiogenic, but all these are isotopes that are radioactive, with half-lives too short to occur primordially. Thus, they are only present as ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
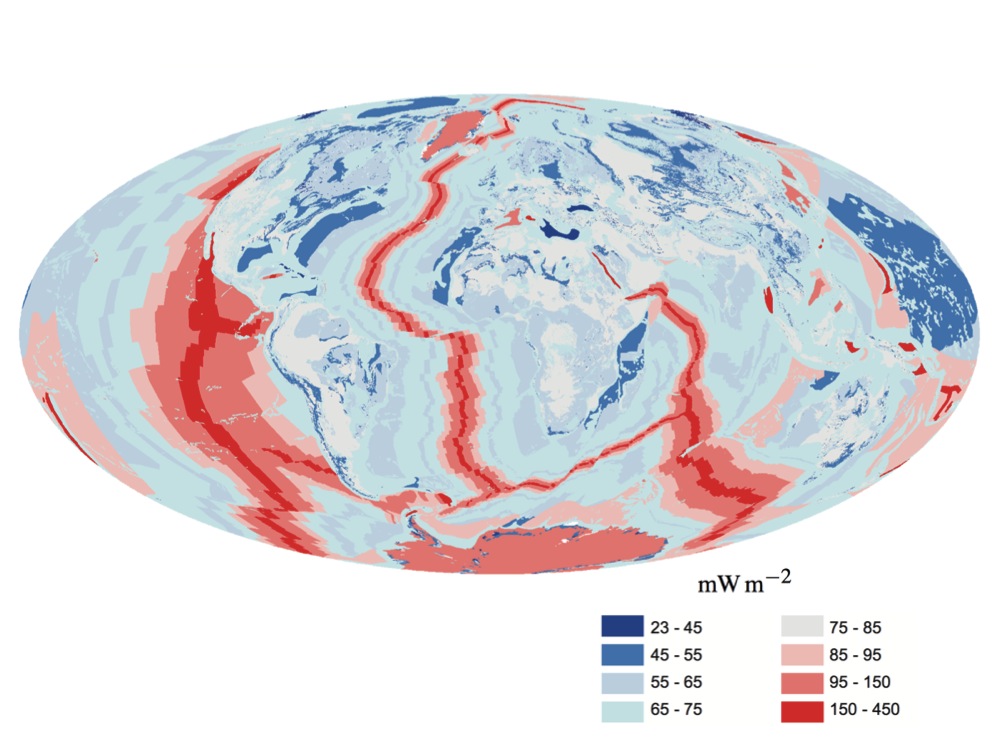
Earth's Internal Heat Budget
Earth's internal heat budget is fundamental to the thermal history of the Earth. The flow of heat from Earth's interior to the surface is estimated at 47±2 terawatts (TW)Davies, J. H., & Davies, D. R. (2010). Earth's surface heat flux. Solid Earth, 1(1), 5–24. and comes from two main sources in roughly equal amounts: the ''radiogenic heat'' produced by the radioactive decay of isotopes in the mantle and crust, and the ''primordial heat'' left over from the formation of Earth. Earth's internal heat travels along geothermal gradients and powers most geological processes. It drives mantle convection, plate tectonics, mountain building, rock metamorphism, and volcanism. Convective heat transfer within the planet's high-temperature metallic core is also theorized to sustain a geodynamo which generates Earth's magnetic field. Despite its geological significance, Earth's interior heat contributes only 0.03% of Earth's total energy budget at the surface, which is dominated by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is, :\delta \ce = \left( \frac -1 \right) \times 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Lead
Lead (82Pb) has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the uranium series (or radium series), the actinium series, and the thorium series, respectively; a fourth decay chain, the neptunium series, terminates with the thallium isotope 205Tl. The three series terminating in lead represent the decay chain products of long-lived primordial 238U, 235U, and 232Th, respectively. However, each of them also occurs, to some extent, as primordial isotopes that were made in supernovae, rather than radiogenically as daughter products. The fixed ratio of lead-204 to the primordial amounts of the other lead isotopes may be used as the baseline to estimate the extra amounts of radiogenic lead present in rocks as a result of decay from uranium and thorium. (See lead–lead dating and uranium–lead dating). The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only significantly radioactive elements that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sands as a by-product of e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state. The word ''nuclide'' was coined by Truman Paul Kohman, Truman P. Kohman in 1947. Kohman defined ''nuclide'' as a "species of atom characterized by the constitution of its nucleus" containing a certain number of neutrons and protons. The term thus originally focused on the nucleus. Nuclides vs isotopes A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, while the isotope concept (grouping all atoms of each element) emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its kinetic isotope effect, effect on chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium–lead Dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routine precisions in the 0.1–1 percent range. The method is usually applied to zircon. This mineral incorporates uranium and thorium atoms into its crystal structure, but strongly rejects lead when forming. As a result, newly-formed zircon deposits will contain no lead, meaning that any lead found in the mineral is radiogenic. Since the exact rate at which uranium decays into lead is known, the current ratio of lead to uranium in a sample of the mineral can be used to reliably determine its age. The method relies on two separate decay chains, the uranium series from 238U to 206Pb, with a half-life of 4.47 billion years and the actinium series from 235U to 207Pb, with a half-life of 710 million years. Decay routes Uranium decays to lead v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through beta decay. A gra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Nuclide
Stable nuclides are nuclides that are not radioactive and so (unlike radionuclides) do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been known to decay using current equipment (see list at the end of this article). Of these 80 elements, 26 have only one stable isotope; they are thus termed monoisotopic. The rest have more than one stable isotope. Tin has ten stable isotopes, the largest number of stable isotopes known for an element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 34 more (total of 286) are known to be radioactive with sufficiently long half-lives (also known) to occur primordially. If the half-life of a nuclide is comparable to, or greater t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |