Magnetochemistry on:
[Wikipedia]
[Google]
[Amazon]
Magnetochemistry is concerned with the magnetic properties of
 A variety of methods are available for the measurement of magnetic susceptibility.
*With the
A variety of methods are available for the measurement of magnetic susceptibility.
*With the
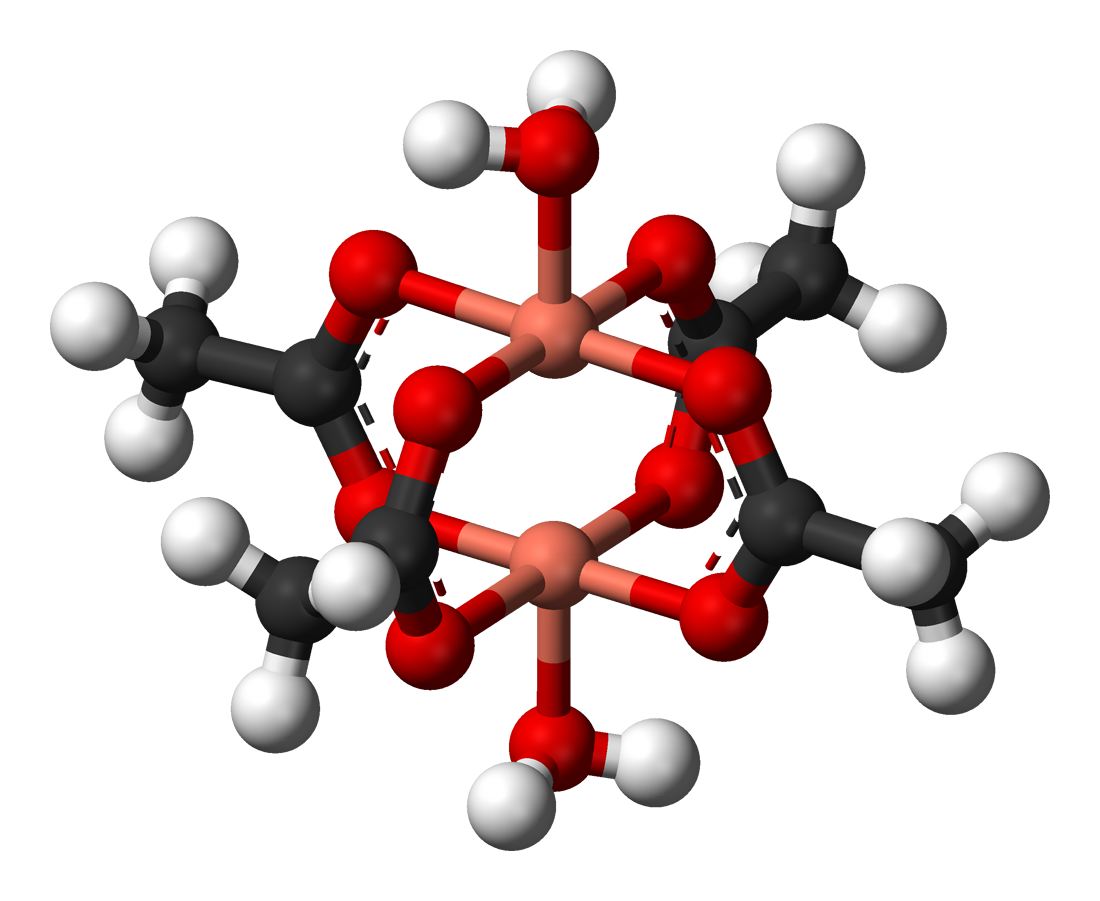

 Exchange interactions occur when the substance is not magnetically dilute and there are interactions between individual magnetic centres. One of the simplest systems to exhibit the result of exchange interactions is crystalline
Exchange interactions occur when the substance is not magnetically dilute and there are interactions between individual magnetic centres. One of the simplest systems to exhibit the result of exchange interactions is crystalline

 According to crystal field theory, the ''d'' orbitals of a transition metal ion in an octahedal complex are split into two groups in a crystal field. If the splitting is large enough to overcome the energy needed to place electrons in the same orbital, with opposite spin, a low-spin complex will result.
:
With one unpaired electron μeff values range from 1.8 to 2.5 μB and with two unpaired electrons the range is 3.18 to 3.3 μB. Note that low-spin complexes of Fe2+ and Co3+ are diamagnetic. Another group of complexes that are diamagnetic are square-planar complexes of d8 ions such as Ni2+ and Rh+ and Au3+.
According to crystal field theory, the ''d'' orbitals of a transition metal ion in an octahedal complex are split into two groups in a crystal field. If the splitting is large enough to overcome the energy needed to place electrons in the same orbital, with opposite spin, a low-spin complex will result.
:
With one unpaired electron μeff values range from 1.8 to 2.5 μB and with two unpaired electrons the range is 3.18 to 3.3 μB. Note that low-spin complexes of Fe2+ and Co3+ are diamagnetic. Another group of complexes that are diamagnetic are square-planar complexes of d8 ions such as Ni2+ and Rh+ and Au3+.

 Very few compounds of main group elements are paramagnetic. Notable examples include:
Very few compounds of main group elements are paramagnetic. Notable examples include:
Online available information resources on magnetochemistry
{{Authority control Magnetism Chemistry
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain ...
s are paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s, spin-orbit coupling cannot be ignored. Exchange interaction
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classic ...
can occur in clusters and infinite lattices, resulting in ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
or ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
depending on the relative orientations of the individual spins.
Magnetic susceptibility
The primary measurement in magnetochemistry is magnetic susceptibility. This measures the strength of interaction on placing the substance in a magnetic field. The volume magnetic susceptibility, represented by the symbol is defined by the relationship : where, is the magnetization of the material (the magnetic dipole moment per unit volume), measured in amperes per meter ( SI units), and is themagnetic field strength
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
, also measured in amperes per meter. Susceptibility is a dimensionless quantity. For chemical applications the molar magnetic susceptibility (χmol) is the preferred quantity. It is measured in m3·mol−1 (SI) or cm3·mol−1 (CGS) and is defined as
:
where ρ is the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
in kg·m−3 (SI) or g·cm−3 (CGS) and ''M'' is molar mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, ...
in kg·mol−1 (SI) or g·mol−1 (CGS).
 A variety of methods are available for the measurement of magnetic susceptibility.
*With the
A variety of methods are available for the measurement of magnetic susceptibility.
*With the Gouy balance
The Gouy balance, invented by the French physicist Louis Georges Gouy, is a device for measuring the magnetic susceptibility of a sample.
Background
Amongst a wide range of interest in optics, Brownian motion, and experimental physics, Gouy als ...
the weight change of the sample is measured with an analytical balance
An analytical balance (or chemical ''balance'') is a class of balance designed to measure small mass in the sub-milligram range. The measuring pan of an analytical balance (0.1 mg resolution or better) is inside a transparent enclosure with do ...
when the sample is placed in a homogeneous magnetic field. The measurements are calibrated
In measurement technology and metrology, calibration is the comparison of measurement values delivered by a device under test with those of a calibration standard of known accuracy. Such a standard could be another measurement device of known ...
against a known standard, such as mercury cobalt thiocyanate, HgCo(NCS)4. Calibration removes the need to know the density of the sample. Variable temperature measurements can be made by placing the sample in a cryostat
A cryostat (from ''cryo'' meaning cold and ''stat'' meaning stable) is a device used to maintain low cryogenic temperatures of samples or devices mounted within the cryostat. Low temperatures may be maintained within a cryostat by using various r ...
between the pole pieces of the magnet.
*The Evans balance
Evans may refer to:
People
* Evans (surname)
* List of people with surname Evans
Places United States
*Evans Island, an island of Alaska
* Evans, Colorado
* Evans, Georgia
* Evans County, Georgia
*Evans, New York
* Evans Mills, New York
*Evans ...
. is a torsion balance
A torsion spring is a spring that works by twisting its end along its axis; that is, a flexible elastic object that stores mechanical energy when it is twisted. When it is twisted, it exerts a torque in the opposite direction, proportional ...
which uses a sample in a fixed position and a variable secondary magnet to bring the magnets back to their initial position. It, too, is calibrated against HgCo(NCS)4.
*With a Faraday balance
A Faraday balance is a device for measuring magnetic susceptibility. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field. Various practical devices are available for the measurement of susceptibility, wh ...
the sample is placed in a magnetic field of constant gradient, and weighed on a torsion balance. This method can yield information on magnetic anisotropy
In condensed matter physics, magnetic anisotropy describes how an object's magnetic properties can be different depending on direction. In the simplest case, there is no preferential direction for an object's magnetic moment. It will respond ...
.
* SQUID is a very sensitive magnetometer.
*For substances in solution NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
may be used to measure susceptibility.
Types of magnetic behaviour
When an isolated atom is placed in a magnetic field there is an interaction because eachelectron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
in the atom behaves like a magnet, that is, the electron has a magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagne ...
. There are two types of interaction.
# Diamagnetism. When placed in a magnetic field the atom becomes magnetically polarized, that is, it develops an induced magnetic moment. The force of the interaction tends to push the atom out of the magnetic field. By convention diamagnetic susceptibility is given a negative sign. Very frequently diamagnetic atoms have no unpaired electrons ''ie'' each electron is paired with another electron in the same atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
. The moments of the two electrons cancel each other out, so the atom has no net magnetic moment. However, for the ion Eu3+ which has six unpaired electrons, the orbital angular momentum cancels out the electron angular momentum, and this ion is diamagnetic at zero Kelvin.
#Paramagnetism. At least one electron is not paired with another. The atom has a permanent magnetic moment. When placed into a magnetic field, the atom is attracted into the field. By convention paramagnetic susceptibility is given a positive sign.
When the atom is present in a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
its magnetic behaviour is modified by its chemical environment. Measurement of the magnetic moment can give useful chemical information.
In certain crystalline materials individual magnetic moments may be aligned with each other (magnetic moment has both magnitude and direction). This gives rise to ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
or ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
. These are properties of the crystal as a whole, of little bearing on chemical properties.
Diamagnetism
Diamagnetism is a universal property of chemical compounds, because all chemical compounds contain electron pairs. A compound in which there are no unpaired electrons is said to be diamagnetic. The effect is weak because it depends on the magnitude of the induced magnetic moment. It depends on the number of electron pairs and the chemical nature of the atoms to which they belong. This means that the effects are additive, and a table of "diamagnetic contributions", orPascal's constants In magnetism, Pascals’ constants are numbers used in the evaluation of the magnetic susceptibilities of coordination compounds. The magnetic susceptibility of a compound is the sum of the paramagnetic susceptibility associated with the unpaired ...
, can be put together. With paramagnetic compounds the observed susceptibility can be adjusted by adding to it the so-called diamagnetic correction, which is the diamagnetic susceptibility calculated with the values from the table.
Paramagnetism
Mechanism and temperature dependence
A metal ion with a single unpaired electron, such as Cu2+, in a coordination complex provides the simplest illustration of the mechanism of paramagnetism. The individual metal ions are kept far apart by the ligands, so that there is no magnetic interaction between them. The system is said to be magnetically dilute. The magnetic dipoles of the atoms point in random directions. When a magnetic field is applied, first-order Zeeman splitting occurs. Atoms with spins aligned to the field slightly outnumber the atoms with non-aligned spins. In the first-order Zeeman effect the energy difference between the two states is proportional to the applied field strength. Denoting the energy difference as Δ''E'', theBoltzmann distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability th ...
gives the ratio of the two populations as , where ''k'' is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
and ''T'' is the temperature in kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phy ...
s. In most cases Δ''E'' is much smaller than ''kT'' and the exponential can be expanded as 1 – Δ''E/kT''. It follows from the presence of 1/''T'' in this expression that the susceptibility is inversely proportional to temperature.
:
This is known as the Curie law and the proportionality constant, ''C'', is known as the Curie constant, whose value, for molar susceptibility, is calculated as
:
where ''N'' is the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining c ...
, ''g'' is the Landé g-factor
In physics, the Landé ''g''-factor is a particular example of a ''g''-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921.
In atomic physics, the Landé '' ...
, and μB is the Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\mat ...
. In this treatment it has been assumed that the electronic ground state is not degenerate, that the magnetic susceptibility is due only to electron spin and that only the ground state is thermally populated.
While some substances obey the Curie law, others obey the Curie-Weiss law.
:
''Tc'' is the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
. The Curie-Weiss law will apply only when the temperature is well above the Curie temperature. At temperatures below the Curie temperature the substance may become ferromagnetic. More complicated behaviour is observed with the heavier transition elements.
Effective magnetic moment
When the Curie law is obeyed, the product of molar susceptibility and temperature is a constant. The effective magnetic moment, μeff is then defined as : Where C has CGS units cm3 mol−1 K, μeff is : Where C has SI units m3 mol−1 K, μeff is : The quantity μeff is effectively dimensionless, but is often stated as in units ofBohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\mat ...
(μB).
For substances that obey the Curie law, the effective magnetic moment is independent of temperature. For other substances μeff is temperature dependent, but the dependence is small if the Curie-Weiss law holds and the Curie temperature is low.
Temperature independent paramagnetism
Compounds which are expected to be diamagnetic may exhibit this kind of weak paramagnetism. It arises from a second-order Zeeman effect in which additional splitting, proportional to the square of the field strength, occurs. It is difficult to observe as the compound inevitably also interacts with the magnetic field in the diamagnetic sense. Nevertheless, data are available for thepermanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a tr ...
ion. It is easier to observe in compounds of the heavier elements, such as uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may ...
compounds.
Exchange interactions

copper(II) acetate
Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2 where AcO− is acetate (). The hydrated derivative, Cu2(OAc)4(H2O)2, which contains one molecule of water for each copper atom, is availab ...
, Cu2(OAc)4(H2O)2. As the formula indicates, it contains two copper(II) ions. The Cu2+ ions are held together by four acetate ligands, each of which binds to both copper ions. Each Cu2+ ion has a d9 electronic configuration, and so should have one unpaired electron. If there were a covalent bond between the copper ions, the electrons would pair up and the compound would be diamagnetic. Instead, there is an exchange interaction in which the spins of the unpaired electrons become partially aligned to each other. In fact two states are created, one with spins parallel and the other with spins opposed. The energy difference between the two states is so small their populations vary significantly with temperature. In consequence the magnetic moment varies with temperature in a sigmoidal pattern. The state with spins opposed has lower energy, so the interaction can be classed as antiferromagnetic in this case. It is believed that this is an example of superexchange, mediated by the oxygen and carbon atoms of the acetate ligands. Other dimers and clusters exhibit exchange behaviour.
Exchange interactions can act over infinite chains in one dimension, planes in two dimensions or over a whole crystal in three dimensions. These are examples of long-range magnetic ordering. They give rise to ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
or ferrimagnetism
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
, depending on the nature and relative orientations of the individual spins.
Compounds at temperatures below the Curie temperature exhibit long-range magnetic order in the form of ferromagnetism. Another critical temperature is the Néel temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
, below which antiferromagnetism occurs. The hexahydrate of nickel chloride, NiCl2·6H2O, has a Néel temperature of 8.3 K. The susceptibility is a maximum at this temperature. Below the Néel temperature the susceptibility decreases and the substance becomes antiferromagnetic.
Complexes of transition metal ions
The effective magnetic moment for a compound containing a transition metal ion with one or more unpaired electrons depends on the total orbital and spinangular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
of the unpaired electrons, and , respectively. "Total" in this context means "vector sum
In mathematics, physics, and engineering, a Euclidean vector or simply a vector (sometimes called a geometric vector or spatial vector) is a geometric object that has magnitude (or length) and direction. Vectors can be added to other vectors a ...
". In the approximation that the electronic states of the metal ions are determined by Russell-Saunders coupling and that spin-orbit coupling is negligible, the magnetic moment is given by
:
Spin-only formula
Orbital angular momentum is generated when an electron in an orbital of a degenerate set of orbitals is moved to another orbital in the set by rotation. In complexes of low symmetry certain rotations are not possible. In that case the orbital angular momentum is said to be "quenched" and is smaller than might be expected (partial quenching), or zero (complete quenching). There is complete quenching in the following cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry. : :legend: t2g, t2 = (dxy, dxz, dyz). eg, e = (dx2–y2, dz2). When orbital angular momentum is completely quenched, and the paramagnetism can be attributed to electron spin alone. The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results. : where ''n'' is the number of unpaired electrons. The spin-only formula is a good first approximation for high-spin complexes of first-rowtransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s.
:
The small deviations from the spin-only formula may result from the neglect of orbital angular momentum or of spin-orbit coupling. For example, tetrahedral d3, d4, d8 and d9 complexes tend to show larger deviations from the spin-only formula than octahedral complexes of the same ion, because "quenching" of the orbital contribution is less effective in the tetrahedral case.
Low-spin complexes
Spin cross-over
When the energy difference between the high-spin and low-spin states is comparable to ''kT'' (''k'' is theBoltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
and ''T'' the temperature) an equilibrium is established between the spin states, involving what have been called "electronic isomers". Tris- dithiocarbamato iron(III), Fe(S2CNR2)3, is a well-documented example. The effective moment varies from a typical d5 low-spin value of 2.25 μB at 80 K to more than 4 μB above 300 K.
2nd and 3rd row transition metals
Crystal field splitting is larger for complexes of the heavier transition metals than for the transition metals discussed above. A consequence of this is that low-spin complexes are much more common. Spin-orbit coupling constants, ζ, are also larger and cannot be ignored, even in elementary treatments. The magnetic behaviour has been summarized, as below, together with an extensive table of data. :Lanthanides and actinides
Russell-Saunders coupling, LS coupling, applies to the lanthanide ions, crystal field effects can be ignored, but spin-orbit coupling is not negligible. Consequently, spin and orbital angular momenta have to be combined : : : and the calculated magnetic moment is given by : : In actinides spin-orbit coupling is strong and the coupling approximates to ''j'' ''j'' coupling. : This means that it is difficult to calculate the effective moment. For example, uranium(IV), f2, in the complex Cl6sup>2− has a measured effective moment of 2.2 μB, which includes a contribution from temperature-independent paramagnetism.Main group elements and organic compounds

 Very few compounds of main group elements are paramagnetic. Notable examples include:
Very few compounds of main group elements are paramagnetic. Notable examples include: oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, O2; nitric oxide, NO; nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the productio ...
, NO2 and chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually ...
, ClO2. In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, compounds with an unpaired electron are said to be free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s. Free radicals, with some exceptions, are short-lived because one free radical will react rapidly with another, so their magnetic properties are difficult to study. However, if the radicals are well separated from each other in a dilute solution in a solid matrix, at low temperature, they can be studied by electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spi ...
(EPR). Such radicals are generated by irradiation. Extensive EPR studies have revealed much about electron delocalization in free radicals. The simulated spectrum of the CH3• radical shows hyperfine splitting
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nuc ...
due to the interaction of the electron with the 3 equivalent hydrogen nuclei, each of which has a spin of 1/2.
Spin label
A spin label (SL) is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynami ...
s are long-lived free radicals which can be inserted into organic molecules so that they can be studied by EPR.
For example, the nitroxide MTSL, a functionalized derivative of TEtra Methyl Piperidine Oxide, TEMPO
In musical terminology, tempo ( Italian, 'time'; plural ''tempos'', or ''tempi'' from the Italian plural) is the speed or pace of a given piece. In classical music, tempo is typically indicated with an instruction at the start of a piece (ofte ...
, is used in site-directed spin labeling Site-directed spin labeling (SDSL) is a technique for investigating the structure and local dynamics of proteins using electron spin resonance. The theory of SDSL is based on the specific reaction of spin labels with amino acids. A spin label's bui ...
.
Applications
Thegadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
ion, Gd3+, has the f7 electronic configuration, with all spins parallel. Compounds of the Gd3+ ion are the most suitable for use as a contrast agent
A contrast agent (or contrast medium) is a substance used to increase the contrast of structures or fluids within the body in medical imaging. Contrast agents absorb or alter external electromagnetism or ultrasound, which is different from radiop ...
for MRI scan
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves ...
s. The magnetic moments of gadolinium compounds are larger than those of any transition metal ion. Gadolinium is
preferred to other lanthanide ions, some of which have larger effective moments, due to its having a non-degenerate electronic ground state.
For many years the nature of oxyhemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
, Hb-O2, was highly controversial. It was found experimentally to be diamagnetic. Deoxy-hemoglobin is generally accepted to be a complex of iron in the +2 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
, that is a d6 system with a high-spin magnetic moment near to the spin-only value of 4.9 μB. It was proposed that the iron is oxidized and the oxygen reduced to superoxide.
:Fe(II)Hb (high-spin) + O2 e(III)Hb2−
Pairing up of electrons from Fe3+ and O2− was then proposed to occur via an exchange mechanism. It has now been shown that in fact the iron(II) changes from high-spin to low-spin when an oxygen molecule donates a pair of electrons to the iron. Whereas in deoxy-hemoglobin the iron atom lies above the plane of the heme, in the low-spin complex the effective ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the catio ...
is reduced and the iron atom lies in the heme plane.
:Fe(II)Hb + O2 e(II)Hb2 (low-spin)
This information has an important bearing on research to find artificial oxygen carriers.
Compounds of gallium(II) were unknown until quite recently. As the atomic number of gallium is an odd number (31), Ga2+ should have an unpaired electron. It was assumed that it would act as a free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
and have a very short lifetime. The non-existence of Ga(II) compounds was part of the so-called inert pair effect The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of ox ...
. When salts of the anion with empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
such as aCl3sup>− were synthesized they were found to be diamagnetic. This implied the formation of a Ga-Ga bond and a dimeric formula, a2Cl6sup>2−.Greenwood&Earnshaw, p. 240
See also
* Magnetic mineralogy * Magnetoelectrochemistry * Magnetic ionic liquid *Spin ice
A spin ice is a magnetic substance that does not have a single minimal-energy state. It has magnetic moments (i.e. "spin") as elementary degrees of freedom which are subject to frustrated interactions. By their nature, these interactions preven ...
*Spin glass
In condensed matter physics, a spin glass is a magnetic state characterized by randomness, besides cooperative behavior in freezing of spins at a temperature called 'freezing temperature' ''Tf''. In ferromagnetic solids, component atoms' magne ...
*Superdiamagnetism
Superdiamagnetism (or perfect diamagnetism) is a phenomenon occurring in certain materials at low temperatures, characterised by the complete absence of magnetic permeability (i.e. a volume magnetic susceptibility \chi_ = −1) and the exclusion ...
, Superparamagnetism, Superferromagnetism
* Single-molecule magnetism
References
Bibliography
* * * * * * *External links
Online available information resources on magnetochemistry
{{Authority control Magnetism Chemistry