|
Kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
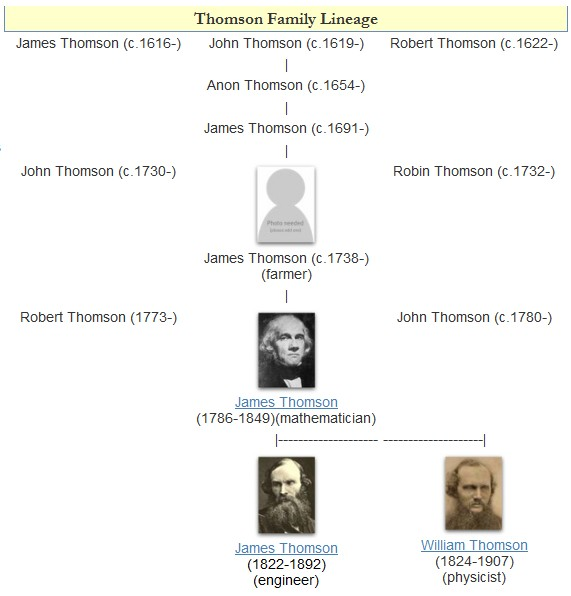
Lord Kelvin
William Thomson, 1st Baron Kelvin (26 June 182417 December 1907), was a British mathematician, Mathematical physics, mathematical physicist and engineer. Born in Belfast, he was the Professor of Natural Philosophy (Glasgow), professor of Natural Philosophy at the University of Glasgow for 53 years, where he undertook significant research on the mathematical analysis of electricity, was instrumental in the formulation of the first and second laws of thermodynamics, and contributed significantly to unifying physics, which was then in its infancy of development as an emerging academic discipline. He received the Royal Society's Copley Medal in 1883 and served as its President of the Royal Society, president from 1890 to 1895. In 1892, he became the first scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in Lord Kelvin's honour. While the existence of a coldest possible temperature, absolute zero, was known before his work, Kelvin d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion. Thermodynamic temperature is typically expressed using the Kelvin scale, where the unit of measurement is the ''kelvin'' (unit symbol: K). The Kelvin scale uses the same degree interval as the Celsius scale but is offset so that 0 K corresponds to absolute zero. For comparison, a temperature of 295 K corresponds to 21.85 °C and 71.33 °F. Another absolute scale of temperature is the Rankine scale, which is based on the Fahrenheit degree interval. Historically, thermodynamic temperature was defined by Lord Kelvin in terms of a macroscopic relation between Work (thermodynamics), thermodynamic work and Heat, heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 °C on the Celsius, Celsius scale, and −459.67 °F on the Fahrenheit scale. The Kelvin and Rankine temperature scales set their zero points at absolute zero by design. This limit can be estimated by extrapolating the ideal gas law to the temperature at which the volume or pressure of a classical gas becomes zero. At absolute zero, there is no thermal motion. However, due to quantum mechanics, quantum effects, the particles still exhibit minimal motion mandated by the Uncertainty principle, Heisenberg uncertainty principle and, for a system of fermions, the Pauli exclusion principle. Even if absolute zero could be achieved, this residual quantum motion would persist. Although absolute zero can be approached, it cannot be reached. Som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celsius
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point of water at 1 atm pressure." (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the closely related Kelvin scale. The degree Celsius (symbol: °C) can refer to a specific point on the Celsius temperature scale or to a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who proposed the first version of it in 1742. The unit was called ''centigrade'' in several languages (from the Latin ''centum'', which means 100, and ''gradus'', which means steps) for many years. In 1948, the International Committee for Weights and Measures renamed it to honor Celsius and also to rem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conversion Of Scales Of Temperature
This is a collection of temperature conversion formulas and comparisons among eight different temperature scales, several of which have long been obsolete. Temperatures on scales that either do not share a numeric zero or are nonlinearly related cannot correctly be mathematically equated (related using the symbol =), and thus temperatures on different scales are more correctly described as corresponding (related using the symbol ≘). Conversion calculator Celsius scale Kelvin scale Fahrenheit scale Rankine scale Delisle scale Newton scale Réaumur scale Rømer scale Comparison values chart Comparison of temperature scales * Normal human body temperature is 36.8 °C ±0.7 °C, or 98.2 °F ±1.3 °F. The commonly given value 98.6 °F is simply the exact conversion of the nineteenth-century German standard of 37 °C. Since it does not list an acceptable range, it could therefore be said to have excess (inv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature Scale
Scale of temperature is a methodology of calibrating the physical quantity temperature in metrology. Empirical scales measure temperature in relation to convenient and stable parameters or reference points, such as the freezing and boiling point of water. Absolute temperature is based on thermodynamic principles: using the lowest possible temperature as the zero point, and selecting a convenient incremental unit. Celsius, Kelvin, and Fahrenheit are common temperature scales. Other scales used throughout history include Rankine, Rømer, Newton, Delisle, Réaumur, Gas mark, Leiden, and Wedgwood. Technical definition The zeroth law of thermodynamics describes thermal equilibrium between thermodynamic systems in form of an equivalence relation. Accordingly, the set of all thermal systems may be divided into a quotient set of equivalence classes, denoted as M, where any element of M collects all systems that are in thermal equilibrium with one another. If the set M h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fahrenheit
The Fahrenheit scale () is a scale of temperature, temperature scale based on one proposed in 1724 by the German-Polish physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit (symbol: °F) as the unit. Several accounts of how he originally defined his scale exist, but the original paper suggests the lower defining point, 0 °F, was established as the freezing temperature of a solution of brine made from a mixture of water, ice, and ammonium chloride (a Salt (chemistry), salt). The other limit established was his best estimate of the average human body temperature, originally set at 90 °F, then 96 °F (about 2.6 °F less than the modern value due to a later redefinition of the scale). For much of the 20th century, the Fahrenheit scale was defined by two fixed points with a 180 °F separation: the temperature at which pure water freezes was defined as 32 °F and the boiling point of water was defined to be 212 °F, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rankine Scale
The Rankine scale ( ) is an absolute scale of thermodynamic temperature named after the University of Glasgow engineer and physicist Macquorn Rankine, who proposed it in 1859. History Similar to the Kelvin scale, which was first proposed in 1848, zero on the Rankine scale is absolute zero, but a temperature difference of one Rankine degree (°R or °Ra) is defined as equal to one Fahrenheit degree, rather than the Celsius degree used on the Kelvin scale. In converting from kelvin to degrees Rankine, 1 K = °Ra or 1 K = 1.8 °Ra. A temperature of 0 K (−273.15 °C; −459.67 °F) is equal to 0 °Ra.B.8 Factors for Units Listed Alphabetically from Usage The Rankine scale is used in engineering systems where ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Base Unit
The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived. The units and their physical quantities are the second for time, the metre (sometimes spelled meter) for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity. The SI base units are a fundamental part of modern metrology, and thus part of the foundation of modern science and technology. The SI base units form a set of mutually independent dimensions as required by dimensional analysis commonly employed in science and technology. The names and symbols of SI base units are written in lowercase, except the symbols of those named after a person, which are written ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |