|
VSEPR
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
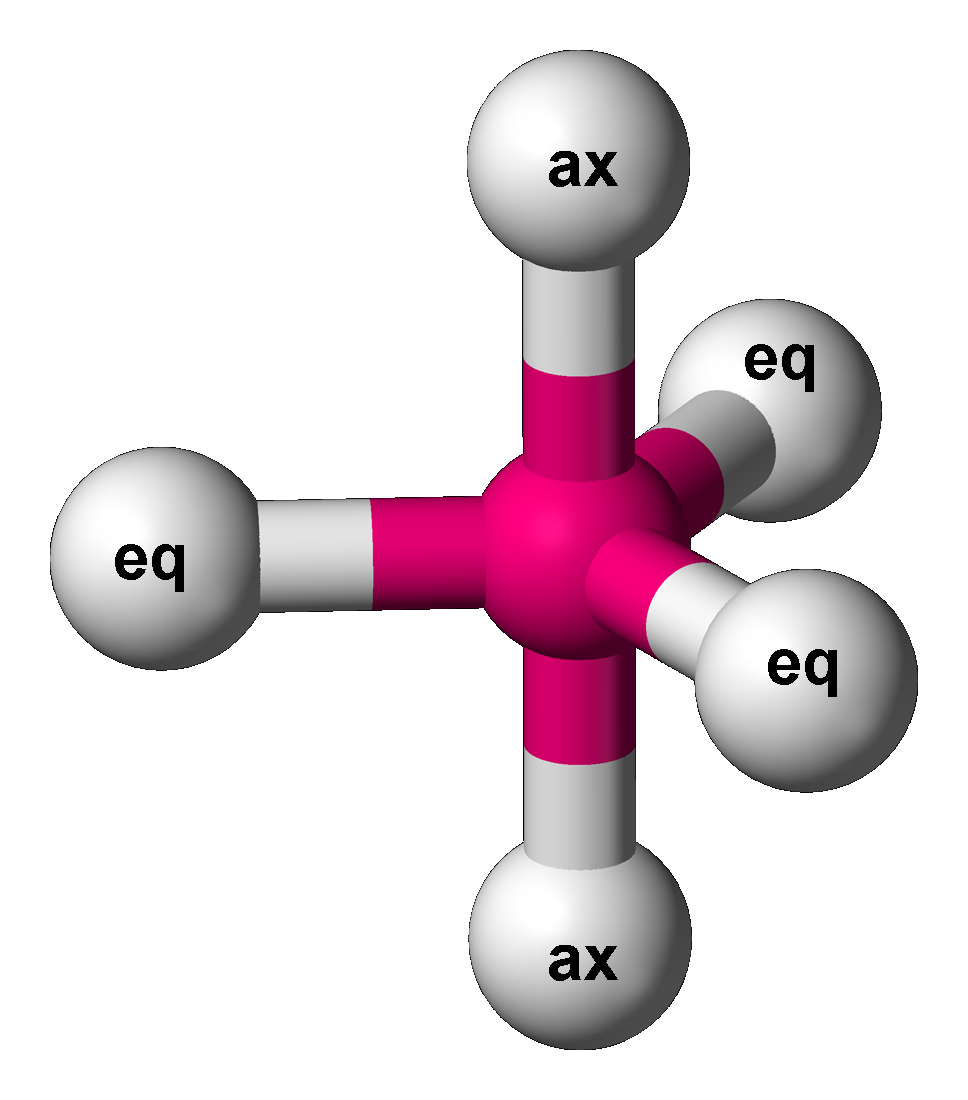
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ronald Gillespie
Ronald James Gillespie, (August 21, 1924 – February 26, 2021) was a British chemist specializing in the field of molecular geometry, who arrived in Canada after accepting an offer that included his own laboratory with new equipment, which post-World War II Britain could not provide. He was responsible for establishing inorganic chemistry education in Canada. He was educated at the University of London obtaining a B.Sc. in 1945, a Ph.D. in 1949 and a D.Sc. in 1957. He was assistant lecturer and then lecturer in the Department of Chemistry at University College London in England from 1950 to 1958. He moved to McMaster University, Hamilton, Ontario, Canada in 1958, passing away on February 26, 2021 at the age of ninety-six years in the nearby town of Dundas, Ontario. He was elected as a Fellow of the Royal Society of Canada in 1965, a Fellow of the Royal Society of London in 1977, and made a member of the Order of Canada in 2007. Gillespie did extensive work on expanding the ide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ronald Sydney Nyholm
Sir Ronald Sydney Nyholm (29 January 1917 – 4 December 1971) was an Australian chemist who was a leading figure in inorganic chemistry in the 1950s and 1960s. Education Born on 29 January 1917 as the fourth in a family of six children. Nyholm's father, Eric Edward Nyholm (1878–1932) was a railway guard. Nyholm's paternal grandfather, Erik Nyholm (1850–1887) was a coppersmith born in Nykarleby in the Swedish-speaking part of Finland, who migrated to Adelaide in 1873. Ronald Nyholm valued his Finnish roots and was particularly proud in his election in 1959 as Corresponding Member of the Finnish Chemical Society. Hailing from the small mining town of Broken Hill, New South Wales, he was early exposed to the role of inorganic chemistry. He attended Burke Ward Public School and Broken Hill High School. Nyholm married Maureen Richardson of Epping, a suburb of Sydney, NSW, at the parish church in Kensington, London on 6 August 1948. After graduating from Broken Hill Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nevil Sidgwick
Nevil Vincent Sidgwick FRS (8 May 1873 – 15 March 1952) was an English theoretical chemist who made significant contributions to the theory of valency and chemical bonding. Biography Sidgwick was born in Park Town, Oxford, the elder of two children of William Carr Sidgwick, lecturer at Oriel College, and Sarah Isabella (née Thompson), descended from a notable family; her uncle was Thomas Perronet Thompson. He was initially educated at Summer Fields School but, after a year, he entered Rugby School in 1886. From there he was elected to an open scholarship in Natural Science at Christ Church, Oxford. He gained a first in 1895, and went on to gain another first in Greats in 1897, a very rare feat. His principal interest, though, was science, and he spent some time in Wilhelm Ostwald’s laboratory in Germany, where he fell ill and had to go home. He returned to Germany in the autumn of 1899, this time in Hans von Pechmann’s lab at the University of Tübingen. His researche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article ''The Atom and the Molecule.'' Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. Although main group eleme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Localization Function
In quantum chemistry, the electron localization function (ELF) is a measure of the likelihood of finding an electron in the neighborhood space of a reference electron located at a given point and with the same spin. Physically, this measures the extent of spatial localization of the reference electron and provides a method for the mapping of electron pair probability in multielectronic systems. ELF's usefulness stems from the observation that it allows electron localization to be analyzed in a chemically intuitive way. For example, the shell structure of heavy atoms is obvious when plotting ELF against the radial distance from the nucleus; the ELF for radon has six clear maxima, whereas the electronic density decreases monotonically and the radially weighted density fails to show all shells. When applied to molecules, an analysis of the ELF shows a clear separation between the core and valence electron, and also shows covalent bonds and lone pairs, in what has been called "a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University College London
, mottoeng = Let all come who by merit deserve the most reward , established = , type = Public research university , endowment = £143 million (2020) , budget = £1.544 billion (2019/20) , chancellor = Anne, Princess Royal(as Chancellor of the University of London) , provost = Michael Spence , head_label = Chair of the council , head = Victor L. L. Chu , free_label = Visitor , free = Sir Geoffrey Vos , academic_staff = 9,100 (2020/21) , administrative_staff = 5,855 (2020/21) , students = () , undergrad = () , postgrad = () , coordinates = , campus = Urban , city = London, England , affiliations = , colours = Purple and blue celeste , nickname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)