In
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, the oxidation state, or oxidation number, is the hypothetical
charge of an atom if all of its
bonds to other atoms are fully
ionic. It describes the degree of
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
(loss of
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s) of an
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
in a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure
ionic bonding
Ionic bonding is a type of chemical bonding that involves the Coulomb's law, electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in io ...
, many
covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s exhibit a strong ionicity, making oxidation state a useful predictor of charge.
The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the
ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on
the choice of
electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. It is nevertheless important in understanding the nomenclature conventions of
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
s. Also, several observations regarding chemical reactions may be explained at a basic level in terms of oxidation states.
Oxidation states are typically represented by
integer
An integer is the number zero (0), a positive natural number (1, 2, 3, ...), or the negation of a positive natural number (−1, −2, −3, ...). The negations or additive inverses of the positive natural numbers are referred to as negative in ...
s which may be positive, zero, or negative. In some cases, the average oxidation state of an element is a fraction, such as for
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
in
magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
(
see below). The highest known oxidation state is reported to be +9, displayed by
iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
in the
tetroxoiridium(IX) cation (). It is predicted that even a +10 oxidation state may be achieved by
platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
in tetroxoplatinum(X), . The lowest oxidation state is −5, as for
boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
in and
gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
in
pentamagnesium digallide ().
In
Stock nomenclature, which is commonly used for inorganic compounds, the oxidation state is represented by a
Roman numeral
Roman numerals are a numeral system that originated in ancient Rome and remained the usual way of writing numbers throughout Europe well into the Late Middle Ages. Numbers are written with combinations of letters from the Latin alphabet, ea ...
placed after the element name inside parentheses or as a superscript after the element symbol, e.g.
Iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when use ...
. The term ''oxidation'' was first used by
Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
to signify the reaction of a substance with
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. Much later, it was realized that the substance, upon being oxidized, loses electrons, and the meaning was extended to include other
reactions in which electrons are lost, regardless of whether oxygen was involved.
The increase in the oxidation state of an atom, through a chemical reaction, is known as oxidation; a decrease in oxidation state is known as a
reduction. Such reactions involve the formal transfer of electrons: a net gain in electrons being a reduction, and a net loss of electrons being oxidation. For pure elements, the oxidation state is zero.
Overview
Oxidation numbers are assigned to elements in a molecule such that the overall sum is zero in a neutral molecule. The number indicates the degree of oxidation of each element caused by molecular bonding. In ionic compounds, the oxidation numbers are the same as the element's ionic charge. Thus for KCl, potassium is assigned +1 and chlorine is assigned −1.
[ The complete set of rules for assigning oxidation numbers are discussed in the following sections.
Oxidation numbers are fundamental to the ]chemical nomenclature
Chemical nomenclature is a set of rules to generate systematic name#In chemistry, systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Appli ...
of ionic compounds. For example, Cu compounds with Cu oxidation state +2 are called ''cupric'' and those with state +1 are ''cuprous''.[Siebring, B. R., Schaff, M. E. (1980). General Chemistry. United States: Wadsworth Publishing Company.]
The oxidation numbers of elements allow predictions of chemical formula and reactions, especially oxidation-reduction reactions.
The oxidation numbers of the most stable chemical compounds follow trends in the periodic table.[Gray, H. B., Haight, G. P. (1967). Basic Principles of Chemistry. Netherlands: W. A. Benjamin.]
IUPAC definition
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) has published a "Comprehensive definition of oxidation state (IUPAC Recommendations 2016)".platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
in , for Pauling and Mulliken scales.
Determination
While introductory levels of chemistry teaching use postulated oxidation states, the IUPAC recommendation
Simple approach without bonding considerations
Introductory chemistry uses postulates: the oxidation state for an element in a chemical formula is calculated from the overall charge and postulated oxidation states for all the other atoms.
A simple example is based on two postulates,
# OS = +1 for hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
# OS = −2 for oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
where OS stands for oxidation state. This approach yields correct oxidation states in oxides and hydroxides of any single element, and in acids such as sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
() or dichromic acid (). Its coverage can be extended either by a list of exceptions or by assigning priority to the postulates. The latter works for hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
() where the priority of rule 1 leaves both oxygens with oxidation state −1.
Additional postulates and their ranking may expand the range of compounds to fit a textbook's scope. As an example, one postulatory algorithm from many possible; in a sequence of decreasing priority:
# An element in a free form has OS = 0.
# In a compound or ion, the sum of the oxidation states equals the total charge of the compound or ion.
# Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
in compounds has OS = −1; this extends to chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
only when not bonded to a lighter halogen, oxygen or nitrogen.
# Group 1 and group 2 metals in compounds have OS = +1 and +2, respectively.
# Hydrogen has OS = +1 but adopts −1 when bonded as a hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
to metals or metalloids.
# Oxygen in compounds has OS = −2 but only when not bonded to oxygen (e.g. in peroxides) or fluorine.
This set of postulates covers oxidation states of fluorides, chlorides, bromides, oxides, hydroxides, and hydrides of any single element. It covers all oxoacids of any central atom (and all their fluoro-, chloro-, and bromo-relatives), as well as salts of such acids with group 1 and 2 metals. It also covers iodides, sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s, and similar simple salts of these metals.
Algorithm of assigning bonds
This algorithm is performed on a Lewis structure (a diagram that shows all valence electrons). Oxidation state equals the charge of an atom after each of its heteronuclear bonds has been assigned to the more electronegative partner of the bond ( except when that partner is a reversibly bonded Lewis-acid ligand) and homonuclear bonds have been divided equally:
: where each "—" represents an electron pair (either shared between two atoms or solely on one atom), and "OS" is the oxidation state as a numerical variable.
After the electrons have been assigned according to the vertical red lines on the formula, the total number of valence electrons that now "belong" to each atom is subtracted from the number of valence electrons of the neutral atom (such as 5 for nitrogen in group 15) to yield that atom's oxidation state.
This example shows the importance of describing the bonding. Its summary formula, , corresponds to two
where each "—" represents an electron pair (either shared between two atoms or solely on one atom), and "OS" is the oxidation state as a numerical variable.
After the electrons have been assigned according to the vertical red lines on the formula, the total number of valence electrons that now "belong" to each atom is subtracted from the number of valence electrons of the neutral atom (such as 5 for nitrogen in group 15) to yield that atom's oxidation state.
This example shows the importance of describing the bonding. Its summary formula, , corresponds to two structural isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The ...
s; the peroxynitrous acid in the above figure and the more stable nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
. With the formula , the simple approach without bonding considerations yields −2 for all three oxygens and +5 for nitrogen, which is correct for nitric acid. For the peroxynitrous acid, however, both oxygens in the O–O bond have OS = −1, and the nitrogen has OS = +3, which requires a structure to understand.
Organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s are treated in a similar manner; exemplified here on functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s occurring in between methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
() and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
():
: Analogously for transition-metal compounds; on the left has a total of 36 valence electrons (18 pairs to be distributed), and hexacarbonylchromium () on the right has 66 valence electrons (33 pairs):
:
Analogously for transition-metal compounds; on the left has a total of 36 valence electrons (18 pairs to be distributed), and hexacarbonylchromium () on the right has 66 valence electrons (33 pairs):
: A key step is drawing the Lewis structure of the molecule (neutral, cationic, anionic): Atom symbols are arranged so that pairs of atoms can be joined by single two-electron bonds as in the molecule (a sort of "skeletal" structure), and the remaining valence electrons are distributed such that sp atoms obtain an octet (duet for hydrogen) with a priority that increases in proportion with electronegativity. In some cases, this leads to alternative formulae that differ in bond orders (the full set of which is called the resonance formulas). Consider the sulfate anion () with 32 valence electrons; 24 from oxygens, 6 from sulfur, 2 of the anion charge obtained from the implied cation. The bond orders to the terminal oxygens do not affect the oxidation state so long as the oxygens have octets. Already the skeletal structure, top left, yields the correct oxidation states, as does the Lewis structure, top right (one of the resonance formulas):
:
A key step is drawing the Lewis structure of the molecule (neutral, cationic, anionic): Atom symbols are arranged so that pairs of atoms can be joined by single two-electron bonds as in the molecule (a sort of "skeletal" structure), and the remaining valence electrons are distributed such that sp atoms obtain an octet (duet for hydrogen) with a priority that increases in proportion with electronegativity. In some cases, this leads to alternative formulae that differ in bond orders (the full set of which is called the resonance formulas). Consider the sulfate anion () with 32 valence electrons; 24 from oxygens, 6 from sulfur, 2 of the anion charge obtained from the implied cation. The bond orders to the terminal oxygens do not affect the oxidation state so long as the oxygens have octets. Already the skeletal structure, top left, yields the correct oxidation states, as does the Lewis structure, top right (one of the resonance formulas):
: The bond-order formula at the bottom is closest to the reality of four equivalent oxygens each having a total bond order of 2. That total includes the bond of order to the implied cation and follows the 8 − ''N'' rule
The bond-order formula at the bottom is closest to the reality of four equivalent oxygens each having a total bond order of 2. That total includes the bond of order to the implied cation and follows the 8 − ''N'' ruleammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
cation of 8 valence electrons (5 from nitrogen, 4 from hydrogens, minus 1 electron for the cation's positive charge):
: Drawing Lewis structures with electron pairs as dashes emphasizes the essential equivalence of bond pairs and lone pairs when counting electrons and moving bonds onto atoms. Structures drawn with electron dot pairs are of course identical in every way:
:
Drawing Lewis structures with electron pairs as dashes emphasizes the essential equivalence of bond pairs and lone pairs when counting electrons and moving bonds onto atoms. Structures drawn with electron dot pairs are of course identical in every way:
:
The algorithm's caveat
The algorithm contains a caveat, which concerns rare cases of transition-metal complexes with a type of ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
that is reversibly bonded as a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
(as an acceptor of the electron pair from the transition metal); termed a "Z-type" ligand in Green's covalent bond classification method. The caveat originates from the simplifying use of electronegativity instead of the MO-based electron allegiance to decide the ionic sign.sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
() as the reversibly-bonded acceptor ligand (released upon heating). The Rh−S bond is therefore extrapolated ionic against Allen electronegativities of rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
and sulfur, yielding oxidation state +1 for rhodium:
:
Algorithm of summing bond orders
This algorithm works on Lewis structures and bond graphs of extended (non-molecular) solids:
Applied to a Lewis structure
An example of a Lewis structure with no formal charge,
: illustrates that, in this algorithm, homonuclear bonds are simply ignored (the bond orders are in blue).
Carbon monoxide exemplifies a Lewis structure with
illustrates that, in this algorithm, homonuclear bonds are simply ignored (the bond orders are in blue).
Carbon monoxide exemplifies a Lewis structure with formal charges
In chemistry, a formal charge (F.C. or ), in the covalent view of Chemical bond, chemical bonding, is the hypothetical electric charge, charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally bet ...
:
: To obtain the oxidation states, the formal charges are summed with the bond-order value taken positively at the carbon and negatively at the oxygen.
Applied to molecular ions, this algorithm considers the actual location of the formal (ionic) charge, as drawn in the Lewis structure. As an example, summing bond orders in the
To obtain the oxidation states, the formal charges are summed with the bond-order value taken positively at the carbon and negatively at the oxygen.
Applied to molecular ions, this algorithm considers the actual location of the formal (ionic) charge, as drawn in the Lewis structure. As an example, summing bond orders in the ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
cation yields −4 at the nitrogen of formal charge +1, with the two numbers adding to the oxidation state of −3:
: The sum of oxidation states in the ion equals its charge (as it equals zero for a neutral molecule).
Also in anions, the formal (ionic) charges have to be considered when nonzero. For sulfate this is exemplified with the skeletal or Lewis structures (top), compared with the bond-order formula of all oxygens equivalent and fulfilling the octet and 8 − ''N'' rules (bottom):
:
The sum of oxidation states in the ion equals its charge (as it equals zero for a neutral molecule).
Also in anions, the formal (ionic) charges have to be considered when nonzero. For sulfate this is exemplified with the skeletal or Lewis structures (top), compared with the bond-order formula of all oxygens equivalent and fulfilling the octet and 8 − ''N'' rules (bottom):
:
Applied to bond graph
A bond graph in solid-state chemistry is a chemical formula of an extended structure, in which direct bonding connectivities are shown. An example is the perovskite, the unit cell of which is drawn on the left and the bond graph (with added numerical values) on the right:
: We see that the oxygen atom bonds to the six nearest rubidium cations, each of which has 4 bonds to the auride anion. The bond graph summarizes these connectivities. The bond orders (also called bond valences) sum up to oxidation states according to the attached sign of the bond's ionic approximation (there are no formal charges in bond graphs).
Determination of oxidation states from a bond graph can be illustrated on ilmenite, . We may ask whether the mineral contains and , or and . Its crystal structure has each metal atom bonded to six oxygens and each of the equivalent oxygens to two
We see that the oxygen atom bonds to the six nearest rubidium cations, each of which has 4 bonds to the auride anion. The bond graph summarizes these connectivities. The bond orders (also called bond valences) sum up to oxidation states according to the attached sign of the bond's ionic approximation (there are no formal charges in bond graphs).
Determination of oxidation states from a bond graph can be illustrated on ilmenite, . We may ask whether the mineral contains and , or and . Its crystal structure has each metal atom bonded to six oxygens and each of the equivalent oxygens to two iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
s and two titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
s, as in the bond graph below. Experimental data show that three metal-oxygen bonds in the octahedron are short and three are long (the metals are off-center). The bond orders (valences), obtained from the bond lengths by the bond valence method, sum up to 2.01 at Fe and 3.99 at Ti; which can be rounded off to oxidation states +2 and +4, respectively:
:
Balancing redox
Oxidation states can be useful for balancing chemical equations for oxidation-reduction (or redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
) reactions, because the changes in the oxidized atoms have to be balanced by the changes in the reduced atoms. For example, in the reaction of acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
with Tollens' reagent to form acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
(shown below), the carbonyl carbon atom changes its oxidation state from +1 to +3 (loses two electrons). This oxidation is balanced by reducing two cations to (gaining two electrons in total).
: An inorganic example is the Bettendorf reaction using tin dichloride () to prove the presence of arsenite ions in a concentrated HCl extract. When arsenic(III) is present, a brown coloration appears forming a dark precipitate of
An inorganic example is the Bettendorf reaction using tin dichloride () to prove the presence of arsenite ions in a concentrated HCl extract. When arsenic(III) is present, a brown coloration appears forming a dark precipitate of arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
, according to the following simplified reaction:
:
Here three tin atoms are oxidized from oxidation state +2 to +4, yielding six electrons that reduce two arsenic atoms from oxidation state +3 to 0. The simple one-line balancing goes as follows: the two redox couples are written down as they react;
:
One tin is oxidized from oxidation state +2 to +4, a two-electron step, hence 2 is written in front of the two arsenic partners. One arsenic is reduced from +3 to 0, a three-electron step, hence 3 goes in front of the two tin partners. An alternative three-line procedure is to write separately the half-reaction
In chemistry, a half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the r ...
s for oxidation and reduction, each balanced with electrons, and then to sum them up such that the electrons cross out. In general, these redox balances (the one-line balance or each half-reaction) need to be checked for the ionic and electron charge sums on both sides of the equation being indeed equal. If they are not equal, suitable ions are added to balance the charges and the non-redox elemental balance.
Appearances
Nominal oxidation states
A nominal oxidation state is a general term with two different definitions:
* Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typi ...
oxidation statesulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
at pH 0 where the electrochemical oxidation state +2 for sulfur puts between S and H2SO3:
:: * Systematic oxidation state is chosen from close alternatives as a pedagogical description. An example is the oxidation state of phosphorus in H3PO3 (structurally diprotic HPO(OH)2) taken nominally as +3, while Allen electronegativities of
* Systematic oxidation state is chosen from close alternatives as a pedagogical description. An example is the oxidation state of phosphorus in H3PO3 (structurally diprotic HPO(OH)2) taken nominally as +3, while Allen electronegativities of phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
suggest +5 by a narrow margin that makes the two alternatives almost equivalent:
:: :Both alternative oxidation numbers for phosphorus make chemical sense, depending on which chemical property or reaction is emphasized. By contrast, a calculated alternative, such as the average (+4) does not.
:Both alternative oxidation numbers for phosphorus make chemical sense, depending on which chemical property or reaction is emphasized. By contrast, a calculated alternative, such as the average (+4) does not.
Ambiguous oxidation states
Lewis formulae are rule-based approximations of chemical reality, as are Allen electronegativities. Still, oxidation states may seem ambiguous when their determination is not straightforward. If only an experiment can determine the oxidation state, the rule-based determination is ambiguous (insufficient). There are also truly dichotomous
A dichotomy () is a partition of a set, partition of a whole (or a set) into two parts (subsets). In other words, this couple of parts must be
* jointly exhaustive: everything must belong to one part or the other, and
* mutually exclusive: nothi ...
values that are decided arbitrarily.
Oxidation-state determination from resonance formulas
Seemingly ambiguous oxidation states are derived from a set of resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
formulas of equal weights for a molecule having heteronuclear bonds where the atom connectivity does not correspond to the number of two-electron bonds dictated by the 8 − ''N'' rule.
A physical measurement is needed to determine oxidation state
* when a non-innocent ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
is present, of hidden or unexpected redox properties that could otherwise be assigned to the central atom. An example is the nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
dithiolate complex, .manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
catecholate, .thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
having two possible oxidation states (bond orders are in blue and formal charges in green):
:: :The S–S distance measurement in
:The S–S distance measurement in thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
is needed to reveal that this bond order is very close to 1, as in the formula on the left.
Ambiguous/arbitrary oxidation states
* when the electronegativity difference between two bonded atoms is very small (as in H3PO3). Two almost equivalent pairs of oxidation states, arbitrarily chosen, are obtained for these atoms.
* when an electronegative p-block atom forms solely homonuclear bonds, the number of which differs from the number of two-electron bonds suggested by rules
Rule or ruling may refer to:
Human activity
* The exercise of political or personal control by someone with authority or power
* Business rule, a rule pertaining to the structure or behavior internal to a business
* School rule, a rule tha ...
. Examples are homonuclear finite chains like (the central nitrogen connects two atoms with four two-electron bonds while only three two-electron bonds are required by the 8 − ''N'' rulepolysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
(where all inner sulfurs form two bonds, fulfilling the 8 − ''N'' rule) follows already from its Lewis structure. :The typical oxidation state of nitrogen in N2O is +1, which also obtains for both nitrogens by a molecular orbital approach.
:The typical oxidation state of nitrogen in N2O is +1, which also obtains for both nitrogens by a molecular orbital approach. :Conversely, formal charges against electronegativities in a Lewis structure decrease the bond order of the corresponding bond. An example is
:Conversely, formal charges against electronegativities in a Lewis structure decrease the bond order of the corresponding bond. An example is carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
with a bond-order estimate of 2.6.
Fractional oxidation states
Fractional oxidation states are often used to represent the average oxidation state of several atoms of the same element in a structure. For example, the formula of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
is , implying an average oxidation state for iron of +.propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
, , has been described as having a carbon oxidation state of −. Again, this is an average value since the structure of the molecule is , with the first and third carbon atoms each having an oxidation state of −3 and the central one −2.
An example with true fractional oxidation states for equivalent atoms is potassium superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
, . The diatomic superoxide ion has an overall charge of −1, so each of its two equivalent oxygen atoms is assigned an oxidation state of −. This ion can be described as a resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
hybrid of two Lewis structures, where each oxygen has an oxidation state of 0 in one structure and −1 in the other.
For the cyclopentadienyl anion , the oxidation state of C is −1 + − = −. The −1 occurs because each carbon is bonded to one hydrogen atom (a less electronegative element), and the − because the total ionic charge of −1 is divided among five equivalent carbons. Again this can be described as a resonance hybrid of five equivalent structures, each having four carbons with oxidation state −1 and one with −2.
:
Finally, fractional oxidation numbers are not used in the chemical nomenclature.lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
atoms.
Elements with multiple oxidation states
Most elements have more than one possible oxidation state. For example, carbon has nine possible integer oxidation states from −4 to +4:
:
Oxidation state in metals
Many compounds with luster and electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
maintain a simple stoichiometric formula, such as the golden TiO, blue-black RuO2 or coppery ReO3, all of obvious oxidation state. Ultimately, assigning the free metallic electrons to one of the bonded atoms is not comprehensive and can yield unusual oxidation states. Examples are the LiPb and ordered alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s, the composition and structure of which are largely determined by atomic size and packing factors. Should oxidation state be needed for redox balancing, it is best set to 0 for all atoms of such an alloy.
List of oxidation states of the elements
This is a list of known oxidation states of the chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s, excluding nonintegral values. The most common states appear in bold. The table is based on that of Greenwood and Earnshaw, with additions noted. Every element exists in oxidation state 0 when it is the pure non-ionized element in any phase, whether monatomic or polyatomic allotrope. The column for oxidation state 0 only shows elements known to exist in oxidation state 0 in compounds.
Early forms (octet rule)
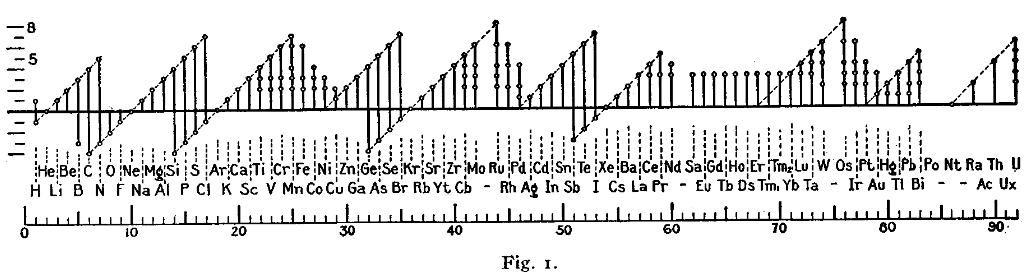
A figure with a similar format was used by Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and metallurgical engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publicatio ...
in 1919 in one of the early papers about the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
. The periodicity of the oxidation states was one of the pieces of evidence that led Langmuir to adopt the rule.
:
Use in nomenclature
The oxidation state in compound naming for transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s and lanthanides and actinides is placed either as a right superscript to the element symbol in a chemical formula, such as FeIII or in parentheses after the name of the element in chemical names, such as iron(III). For example, is named iron(III) sulfate and its formula can be shown as Fe. This is because a sulfate ion has a charge of −2, so each iron atom takes a charge of +3.
History of the oxidation state concept
Early days
Oxidation itself was first studied by Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
, who defined it as the result of reactions with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(hence the name). The term has since been generalized to imply a ''formal'' loss of electrons. Oxidation states, called ''oxidation grades'' by Friedrich Wöhler in 1835, were one of the intellectual stepping stones that Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
used to derive the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. William B. Jensen gives an overview of the history up to 1938.
Use in nomenclature
When it was realized that some metals form two different binary compounds with the same nonmetal, the two compounds were often distinguished by using the ending ''-ic'' for the higher metal oxidation state and the ending ''-ous'' for the lower. For example, FeCl3 is ferric chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated f ...
and FeCl2 is ferrous chloride. This system is not very satisfactory (although sometimes still used) because different metals have different oxidation states which have to be learned: ferric and ferrous are +3 and +2 respectively, but cupric and cuprous are +2 and +1, and stannic and stannous are +4 and +2. Also, there was no allowance for metals with more than two oxidation states, such as vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
with oxidation states +2, +3, +4, and +5.IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
in 1940. Thus, FeCl2 was written as iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water ...
rather than ferrous chloride. The Roman numeral II at the central atom came to be called the " Stock number" (now an obsolete term), and its value was obtained as a charge at the central atom after removing its ligands along with the electron pairs they shared with it.
Development towards the current concept
The term "oxidation state" in English chemical literature was popularized by Wendell Mitchell Latimer in his 1938 book about electrochemical potentials. He used it for the value (synonymous with the German term ''Wertigkeit'') previously termed "valence", "polar valence" or "polar number" in English, or "oxidation stage" or indeed the "state of oxidation". Since 1938, the term "oxidation state" has been connected with electrochemical potentials and electrons exchanged in redox couples participating in redox reactions. By 1948, IUPAC used the 1940 nomenclature rules with the term "oxidation state", instead of the originalLinus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
proposed that oxidation number could be determined by extrapolating bonds to being completely ionic in the direction of electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
. A full acceptance of this suggestion was complicated by the fact that the Pauling electronegativities as such depend on the oxidation state and that they may lead to unusual values of oxidation states for some transition metals. In 1990 IUPAC resorted to a postulatory (rule-based) method to determine the oxidation state.ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
"coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es. This situation and the lack of a real single definition generated numerous debates about the meaning of oxidation state, suggestions about methods to obtain it and definitions of it. To resolve the issue, an IUPAC project (2008-040-1-200) was started in 2008 on the "Comprehensive Definition of Oxidation State", and was concluded by two reports
See also
* Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
* Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
* Atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
* Atomic shell
* Quantum numbers
In Quantum mechanics, quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.
To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditi ...
** Azimuthal quantum number
** Principal quantum number
** Magnetic quantum number
** Spin quantum number
In physics and chemistry, the spin quantum number is a quantum number (designated ) that describes the intrinsic angular momentum (or spin angular momentum, or simply ''spin'') of an electron or other particle. It has the same value for all ...
* Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
** Wiswesser's rule
* Ionization energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, Ion, positive ion, or molecule. The first ionization energy is quantitatively expressed as
: ...
* Electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
This differs by si ...
* Ionic potential
* Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
** Cations and Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
** Polyatomic ions
* Covalent bonding
* Metallic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be desc ...
* Hybridization
References
{{Authority control
Chemical nomenclature
Chemical properties
Coordination chemistry
Dimensionless numbers of chemistry
Redox
