|
Peroxynitrous Acid
Peroxynitrous acid (HNO3) is a reactive nitrogen species (RNS). It is the conjugate acid of peroxynitrite (ONOO−). It has a p''K''a of approximately 6.8. It is formed ''in vivo'' from the diffusion-controlled reaction of nitrogen monoxide (ON•) and superoxide (). It is an isomer of nitric acid and isomerises with a rate constant of ''k'' = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed. It oxidises and nitrates aromatic compounds in low yield. The mechanism may involve a complex between the aromatic compound and ONOOH, and a transition from the ''cis''- to the ''trans''-configuration of ONOOH. Peroxynitrous acid is also important in atmospheric chemistry Atmospheric chemistry is a branch of atmospheric science that studies the chemistry of the Earth's atmosphere and that of other planets. This multidisciplinary approach of research draws on environmental chemistry, physics, meteorology, comput .... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, Preparation Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide: : It is prepared by the reaction of hydrogen peroxide with nitrite: : H2O2 + → ONOO− + H2O Its presence is indicated by the absorbance at 302 nm (pH 12, ''ε''302 = 1670 M−1 cm−1). Reactions Peroxynitrite is weakly basic with a p''K''a of ~6.8. It is reactive toward DNA and proteins. ONOO− reacts nucleophilically with carbon dioxide Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma .... ''In vivo'', the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitroxyl
Nitroxyl (common name) or azanone (IUPAC name) is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in solution. Its conjugate base, NO−, the nitroxide anion, is the reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. The bond dissociation energy of H−NO is , which is unusually weak for a bond to the hydrogen atom. Generation Nitroxyl is produced from the reagents Angeli's salt (Na2N2O3) and Piloty's acid (PhSO2NHOH). Other notable studies on the production of HNO exploit cycloadducts of acyl nitroso species, which are known to decompose via hydrolysis to HNO and acyl acid. Upon photolysis these compounds release the acyl nitroso species which then further decompose. HNO is generated via organic oxidation of cyclohexanone oxime with lead tetraacetate to form 1-nitrosocyclohexyl acetate: This compound can be hydrolyzed under basic conditions in a phosphate buffer to HNO, acetic acid, and cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrous Acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite () salts. It was discovered by Carl Wilhelm Scheele, who called it " phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes. Structure In the gas phase, the planar nitrous acid molecule can adopt both a ''syn'' and an ''anti'' form. The ''anti'' form predominates at room temperature, and IR measurements indicate it is more stable by around 2.3 kJ/mol. p. 462. Image:Trans-nitrous-acid-2D-dimensions.png , Dimensions of the ''anti'' form(from the microwave spectrum) Image:Trans-nitrous-acid-3D-balls.png , Model of the ''anti'' form Image:Cis-nitrous-acid-3D-balls.png , ''syn'' form Preparation and decomposition Free, gaseous nitrous acid is unstable, rapidly disproportionating to nitric oxides: :2 HNO2 → NO2 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrosyl Bromide
Nitrosyl bromide is the chemical compound with the chemical formula NOBr. It is a red gas with a condensation point just below room temperature. It reacts with water. Nitrosyl bromide can be formed by the reversible reaction of nitric oxide with bromine. This reaction is of interest as it is one of very few third-order homogeneous gas reactions. NOBr is prone to photodissociation at standard pressure and temperature. :2 NO + Br2 ⇌ 2 NOBr Another way to make it is by way of nitrogen dioxide reacting with potassium bromide. :2NO2 + KBr → BrNO + KNO3 Dissociation kinetics The bond breaking of the chemical can be done with photolysis using a light to separate the molecules that are present. Another to separate nitrosyl bromide into NO and Br or Br2 is by having excess of NO which then the experiment will follow first order kinetics. This reverse rate constant was calculated to be kr = 2.29 ± 0.33 x 10-21 cm3 /molecules With excess Br2 plus NO the reaction follows third order ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
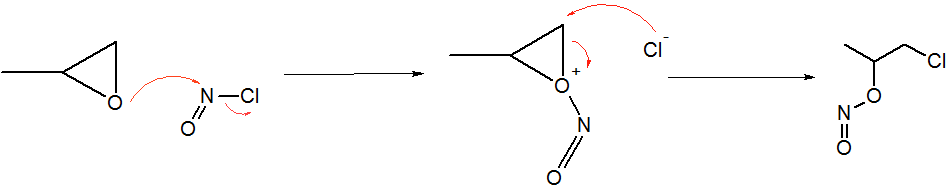
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrosyl Fluoride
Nitrosyl fluoride ( N O F) is a covalently bonded nitrosyl compound. Physical properties The compound is a colorless gas, with bent molecular shape. The VSEPR model explains this geometry via a lone-pair of electrons on the nitrogen atom. Chemistry Nitrosyl fluoride is typically produced by direct reaction of nitric oxide and fluorine, although halogenation with a perfluorinated metal salt is also possible. The compound is a highly reactive fluorinating agent that converts many metals to their fluorides, releasing nitric oxide in the process: :''n'' NOF + M → MF''n'' + ''n'' NO For this reason, aqueous NOF solutions are, like aqua regia, powerful solvents for metals. Absent an oxidizable metal, NOF reacts with water to form nitrous acid, which then disproportionates to nitric acid: :NOF + H2O → HNO2 + HF :3 HNO2 → HNO3 + 2 NO + H2O These reactions occur in both acidic and basic solutions. Nitrosyl fluoride also forms salt-like adducts with Lewis-aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reactive Nitrogen Species
Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•−) produced via the enzymatic activity of inducible nitric oxide synthase 2 (Nitric oxide synthase 2A, NOS2) and NADPH oxidase respectively. NOS2 is expressed primarily in macrophages after induction by cytokines and microbial products, notably interferon-gamma (IFN-γ) and lipopolysaccharide (LPS). Reactive nitrogen species act together with reactive oxygen species (ROS) to damage biological cells, cells, causing nitrosative stress. Therefore, these two species are often collectively referred to as ROS/RNS. Reactive nitrogen species are also continuously produced in plants as by-products of aerobic metabolism or in response to stress. Types RNS are produced in animals starting with the reaction of nitric oxide (•NO) with superoxide (O2•−) to form peroxynitrite (ONOO−): * •NO (nitric oxide) + O2•− (superoxide) → ONOO− (peroxynitrit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: \text + \text \; \ce \; \text + \text Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which said that any compound that can give a proton to another compound is an acid, and the compound that receives the proton is a base. A proton is a subatomic particle in the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, Preparation Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide: : It is prepared by the reaction of hydrogen peroxide with nitrite: : H2O2 + → ONOO− + H2O Its presence is indicated by the absorbance at 302 nm (pH 12, ''ε''302 = 1670 M−1 cm−1). Reactions Peroxynitrite is weakly basic with a p''K''a of ~6.8. It is reactive toward DNA and proteins. ONOO− reacts nucleophilically with carbon dioxide Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma .... ''In vivo'', the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen , which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide". Salts Superoxide forms salts with alkali metals and alkaline earth metals. The salts sodium superoxide (), potassium superoxide (), rubidium superoxide () and caesium superoxide () are prepared by the reaction of with the respect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |