noble gases on:
[Wikipedia]
[Google]
[Amazon]
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of group 18 of the
 Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the chromosphere of the Sun, and named it
Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the chromosphere of the Sun, and named it
 The noble gas
The noble gas
 The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. They were once labeled '' group 0'' in the
The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. They were once labeled '' group 0'' in the
 The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in
The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in 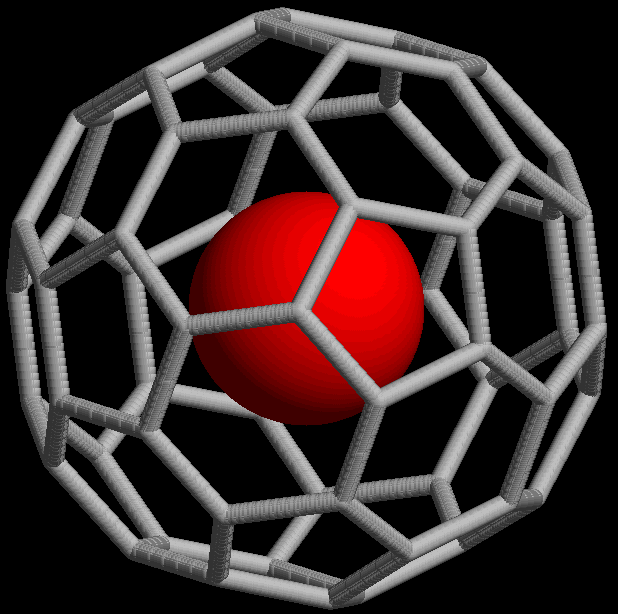 Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when , a spherical molecule consisting of 60
Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when , a spherical molecule consisting of 60  Noble gas compounds such as xenon difluoride () are considered to be hypervalent because they violate the octet rule. Bonding in such compounds can be explained using a three-center four-electron bond model. This model, first proposed in 1951, considers bonding of three collinear atoms. For example, bonding in is described by a set of three molecular orbitals (MOs) derived from p-orbitals on each atom. Bonding results from the combination of a filled p-orbital from Xe with one half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding orbital. The highest occupied molecular orbital is localized on the two terminal atoms. This represents a localization of charge that is facilitated by the high electronegativity of fluorine.
The chemistry of the heavier noble gases, krypton and xenon, are well established. The chemistry of the lighter ones, argon and helium, is still at an early stage, while a neon compound is yet to be identified.
Noble gas compounds such as xenon difluoride () are considered to be hypervalent because they violate the octet rule. Bonding in such compounds can be explained using a three-center four-electron bond model. This model, first proposed in 1951, considers bonding of three collinear atoms. For example, bonding in is described by a set of three molecular orbitals (MOs) derived from p-orbitals on each atom. Bonding results from the combination of a filled p-orbital from Xe with one half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding orbital. The highest occupied molecular orbital is localized on the two terminal atoms. This represents a localization of charge that is facilitated by the high electronegativity of fluorine.
The chemistry of the heavier noble gases, krypton and xenon, are well established. The chemistry of the lighter ones, argon and helium, is still at an early stage, while a neon compound is yet to be identified.
For large-scale use, helium is extracted by fractional distillation from natural gas, which can contain up to 7% helium.
 Noble gases have very low boiling and melting points, which makes them useful as cryogenic refrigerants. In particular, liquid helium, which boils at , is used for superconducting magnets, such as those needed in nuclear magnetic resonance imaging and nuclear magnetic resonance. Liquid neon, although it does not reach temperatures as low as liquid helium, also finds use in cryogenics because it has over 40 times more refrigerating capacity than liquid helium and over three times more than liquid hydrogen.
Helium is used as a component of breathing gases to replace nitrogen, due its low
Noble gases have very low boiling and melting points, which makes them useful as cryogenic refrigerants. In particular, liquid helium, which boils at , is used for superconducting magnets, such as those needed in nuclear magnetic resonance imaging and nuclear magnetic resonance. Liquid neon, although it does not reach temperatures as low as liquid helium, also finds use in cryogenics because it has over 40 times more refrigerating capacity than liquid helium and over three times more than liquid hydrogen.
Helium is used as a component of breathing gases to replace nitrogen, due its low  Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a lifting gas in blimps and balloons: despite an 8.6% decrease in buoyancy compared to hydrogen, helium is not combustible.
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediates, by trapping them in an inert matrix at very low temperatures. Helium is used as the carrier medium in gas chromatography, as a filler gas for
Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a lifting gas in blimps and balloons: despite an 8.6% decrease in buoyancy compared to hydrogen, helium is not combustible.
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediates, by trapping them in an inert matrix at very low temperatures. Helium is used as the carrier medium in gas chromatography, as a filler gas for  Noble gases are commonly used in
Noble gases are commonly used in
 Giggenbach bottles - Giggenbach bottles are evacuated glass flasks with a Teflon stopcock, used for sampling and storing gases. They require pre-evacuation before sampling, as noble gases accumulate in the headspace. These bottles were first invented and distributed by a Werner F. Giggenbach, a German chemist.
Giggenbach bottles - Giggenbach bottles are evacuated glass flasks with a Teflon stopcock, used for sampling and storing gases. They require pre-evacuation before sampling, as noble gases accumulate in the headspace. These bottles were first invented and distributed by a Werner F. Giggenbach, a German chemist.
miniRuedi
, which can analyze noble gases with an analytical uncertainty of 1-3% using low-cost vacuum systems and quadrupole mass analyzers.
periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
: helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
(He), neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
(Ne), argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
(Ar), krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
(Kr), xenon (Xe), radon (Rn) and, in some cases, oganesson
Oganesson is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint ...
(Og). Under standard conditions, the first six of these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points. The properties of oganesson are uncertain.
The intermolecular force between noble gas atoms is the very weak London dispersion force, so their boiling points are all cryogenic, below .
The noble gases' inertness, or tendency not to react with other chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
s, results from their electron configuration: their outer shell of valence electrons is "full", giving them little tendency to participate in chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s. Only a few hundred noble gas compounds are known to exist. The inertness of noble gases makes them useful whenever chemical reactions are unwanted. For example, argon is used as a shielding gas in welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, primarily by using high temperature to melting, melt the parts together and allow them to cool, causing Fusion welding, fusion. Co ...
and as a filler gas in incandescent light bulbs. Helium is used to provide buoyancy in blimps and balloons. Helium and neon are also used as refrigerants due to their low boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s. Industrial quantities of the noble gases, except for radon, are obtained by separating them from air using the methods of liquefaction of gases and fractional distillation. Helium is also a byproduct of the mining of natural gas. Radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds.
The seventh member of group 18 is oganesson, an unstable synthetic element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it i ...
whose chemistry is still uncertain because only five very short-lived atoms (t1/2 = 0.69 ms) have ever been synthesized (). IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
uses the term "noble gas" interchangeably with "group 18" and thus includes oganesson; however, due to relativistic effects, oganesson is predicted to be a solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
under standard conditions and reactive enough not to qualify functionally as "noble".
History
''Noble gas'' is translated from the German noun , first used in 1900 by Hugo Erdmann to indicate their extremely low level of reactivity. The name makes an analogy to the term " noble metals", which also have low reactivity. The noble gases have also been referred to as '' inert gases'', but this label is deprecated as many noble gas compounds are now known. ''Rare gases'' is another term that was used, but this is also inaccurate becauseargon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
forms a fairly considerable part (0.94% by volume, 1.3% by mass) of the Earth's atmosphere due to decay of radioactive potassium-40.
 Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the chromosphere of the Sun, and named it
Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the chromosphere of the Sun, and named it helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
after the Greek word for the Sun, (). No chemical analysis was possible at the time, but helium was later found to be a noble gas. Before them, in 1784, the English chemist and physicist Henry Cavendish had discovered that air contains a small proportion of a substance less reactive than nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. A century later, in 1895, Lord Rayleigh discovered that samples of nitrogen from the air were of a different density than nitrogen resulting from chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s. Along with Scottish scientist William Ramsay at University College, London, Lord Rayleigh theorized that the nitrogen extracted from air was mixed with another gas, leading to an experiment that successfully isolated a new element, argon, from the Greek word (, "idle" or "lazy"). With this discovery, they realized an entire class of gases was missing from the periodic table. During his search for argon, Ramsay also managed to isolate helium for the first time while heating cleveite, a mineral. In 1902, having accepted the evidence for the elements helium and argon, Dmitri Mendeleev included these noble gases as group 0 in his arrangement of the elements, which would later become the periodic table.
Ramsay continued his search for these gases using the method of fractional distillation to separate liquid air into several components. In 1898, he discovered the elements krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
, neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
, and xenon, and named them after the Greek words (, "hidden"), (, "new"), and (, "stranger"), respectively. Radon was first identified in 1898 by Friedrich Ernst Dorn, and was named ''radium emanation'', but was not considered a noble gas until 1904 when its characteristics were found to be similar to those of other noble gases. Rayleigh and Ramsay received the 1904 Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
s in Physics and in Chemistry, respectively, for their discovery of the noble gases; in the words of J. E. Cederblom, then president of the Royal Swedish Academy of Sciences
The Royal Swedish Academy of Sciences () is one of the Swedish Royal Academies, royal academies of Sweden. Founded on 2 June 1739, it is an independent, non-governmental scientific organization that takes special responsibility for promoting nat ...
, "the discovery of an entirely new group of elements, of which no single representative had been known with any certainty, is something utterly unique in the history of chemistry, being intrinsically an advance in science of peculiar significance".
The discovery of the noble gases aided in the development of a general understanding of atomic structure. In 1895, French chemist Henri Moissan attempted to form a reaction between fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
, the most electronegative element, and argon, one of the noble gases, but failed. Scientists were unable to prepare compounds of argon until the end of the 20th century, but these attempts helped to develop new theories of atomic structure. Learning from these experiments, Danish physicist Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
proposed in 1913 that the electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in atoms are arranged in shells surrounding the nucleus, and that for all noble gases except helium the outermost shell always contains eight electrons. In 1916, Gilbert N. Lewis formulated the '' octet rule'', which concluded an octet of electrons in the outer shell was the most stable arrangement for any atom; this arrangement caused them to be unreactive with other elements since they did not require any more electrons to complete their outer shell.
In 1962, Neil Bartlett discovered the first chemical compound of a noble gas, xenon hexafluoroplatinate. Compounds of other noble gases were discovered soon after: in 1962 for radon, radon difluoride (), which was identified by radiotracer techniques and in 1963 for krypton, krypton difluoride (). The first stable compound of argon was reported in 2000 when argon fluorohydride (HArF) was formed at a temperature of .
In October 2006, scientists from the Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory successfully created synthetically oganesson
Oganesson is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint ...
, the seventh element in group 18, by bombarding californium with calcium.
Physical and atomic properties
The noble gases have weak interatomic force, and consequently have very low melting andboiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s. They are all monatomic gases under standard conditions, including the elements with larger atomic masses than many normally solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
elements. Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
has several unique qualities when compared with other elements: its boiling point at 1 atm is lower than those of any other known substance; it is the only element known to exhibit superfluidity; and, it is the only element that cannot be solidified by cooling at atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
(an effect explained by quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
as its zero point energy is too high to permit freezing) – a pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
of must be applied at a temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
of to convert it to a solid while a pressure of about is required at room temperature. The noble gases up to xenon have multiple stable isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s; krypton and xenon also have naturally occurring radioisotopes, namely 78Kr, 124Xe, and 136Xe, all have very long lives (> 1021 years) and can undergo double electron capture or double beta decay. Radon has no stable isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are no ...
s; its longest-lived isotope, 222Rn, has a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 3.8 days and decays to form helium and polonium, which ultimately decays to lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
. Oganesson also has no stable isotopes, and its only known isotope 294Og is very short-lived (half-life 0.7 ms). Melting and boiling points increase going down the group.
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s, like atoms in most groups, increase steadily in atomic radius from one period to the next due to the increasing number of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s. The size of the atom is related to several properties. For example, the ionization potential decreases with an increasing radius because the valence electrons in the larger noble gases are farther away from the nucleus and are therefore not held as tightly together by the atom. Noble gases have the largest ionization potential among the elements of each period, which reflects the stability of their electron configuration and is related to their relative lack of chemical reactivity. Some of the heavier noble gases, however, have ionization potentials small enough to be comparable to those of other elements and molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s. It was the insight that xenon has an ionization potential similar to that of the oxygen molecule that led Bartlett to attempt oxidizing xenon using platinum hexafluoride, an oxidizing agent known to be strong enough to react with oxygen. Noble gases cannot accept an electron to form stable anions; that is, they have a negative electron affinity.
The macroscopic physical properties of the noble gases are dominated by the weak van der Waals forces
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical ele ...
between the atoms. The attractive force
In physics, a force is an influence that can cause an Physical object, object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the Magnitu ...
increases with the size of the atom as a result of the increase in polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of particles with an electric charge. When subject to an elect ...
and the decrease in ionization potential. This results in systematic group trends: as one goes down group 18, the atomic radius increases, and with it the interatomic forces increase, resulting in an increasing melting point, boiling point, enthalpy of vaporization, and solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
. The increase in density is due to the increase in atomic mass.
The noble gases are nearly ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is ...
es under standard conditions, but their deviations from the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
provided important clues for the study of intermolecular interactions. The Lennard-Jones potential, often used to model intermolecular interactions, was deduced in 1924 by John Lennard-Jones from experimental data on argon before the development of quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
provided the tools for understanding intermolecular forces from first principles. The theoretical analysis of these interactions became tractable because the noble gases are monatomic and the atoms spherical, which means that the interaction between the atoms is independent of direction, or isotropic.
Chemical properties
periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
because it was believed they had a valence of zero, meaning their atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s cannot combine with those of other elements to form compounds. However, it was later discovered some do indeed form compounds, causing this label to fall into disuse.
Electron configuration
Like other groups, the members of thisfamily
Family (from ) is a Social group, group of people related either by consanguinity (by recognized birth) or Affinity (law), affinity (by marriage or other relationship). It forms the basis for social order. Ideally, families offer predictabili ...
show patterns in its electron configuration, especially the outermost shells resulting in trends in chemical behavior:
The noble gases have full valence electron shells. Valence electrons are the outermost electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s of an atom and are normally the only electrons that participate in chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
ing. Atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s and have little tendency to gain or lose electrons. However, heavier noble gases such as radon are held less firmly together by electromagnetic force than lighter noble gases such as helium, making it easier to remove outer electrons from heavy noble gases.
As a result of a full shell, the noble gases can be used in conjunction with the electron configuration notation to form the ''noble gas notation''. To do this, the nearest noble gas that precedes the element in question is written first, and then the electron configuration is continued from that point forward. For example, the electron notation of
phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
is , while the noble gas notation is . This more compact notation makes it easier to identify elements, and is shorter than writing out the full notation of atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s.
The noble gases cross the boundary between blocks—helium is an s-element whereas the rest of members are p-elements—which is unusual among the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
groups. All other IUPAC groups contain elements from ''one'' block each. This causes some inconsistencies in trends across the table, and on those grounds some chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
s have proposed that helium should be moved to group 2 to be with other s2 elements, but this change has not generally been adopted.
Compounds
 The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in
The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933, Linus Pauling predicted that the heavier noble gases could form compounds with fluorine and oxygen. He predicted the existence of krypton hexafluoride () and xenon hexafluoride () and speculated that xenon octafluoride () might exist as an unstable compound, and suggested that xenic acid could form perxenate salts. These predictions were shown to be generally accurate, except that is now thought to be both thermodynamically and kinetically unstable.
Xenon compounds are the most numerous of the noble gas compounds that have been formed. Most of them have the xenon atom in the oxidation state of +2, +4, +6, or +8 bonded to highly electronegative atoms such as fluorine or oxygen, as in xenon difluoride (), xenon tetrafluoride (), xenon hexafluoride (), xenon tetroxide (), and sodium perxenate (). Xenon reacts with fluorine to form numerous xenon fluorides according to the following equations:
::Xe + F2 → XeF2
::Xe + 2F2 → XeF4
::Xe + 3F2 → XeF6
Some of these compounds have found use in chemical synthesis as oxidizing agents; , in particular, is commercially available and can be used as a fluorinating agent. As of 2007, about five hundred compounds of xenon bonded to other elements have been identified, including organoxenon compounds (containing xenon bonded to carbon), and xenon bonded to nitrogen, chlorine, gold, mercury, and xenon itself. Compounds of xenon bound to boron, hydrogen, bromine, iodine, beryllium, sulphur, titanium, copper, and silver have also been observed but only at low temperatures in noble gas matrices, or in supersonic noble gas jets.
Radon is more reactive than xenon, and forms chemical bonds more easily than xenon does. However, due to the high radioactivity and short half-life of radon isotopes, only a few fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
s and oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s of radon have been formed in practice. Radon goes further towards metallic behavior than xenon; the difluoride RnF2 is highly ionic, and cationic Rn2+ is formed in halogen fluoride solutions. For this reason, kinetic hindrance makes it difficult to oxidize radon beyond the +2 state. Only tracer experiments appear to have succeeded in doing so, probably forming RnF4, RnF6, and RnO3.
Krypton is less reactive than xenon, but several compounds have been reported with krypton in the oxidation state of +2. Krypton difluoride is the most notable and easily characterized. Under extreme conditions, krypton reacts with fluorine to form KrF2 according to the following equation:
::Kr + F2 → KrF2
Compounds in which krypton forms a single bond to nitrogen and oxygen have also been characterized, but are only stable below and respectively.
Krypton atoms chemically bound to other nonmetals (hydrogen, chlorine, carbon) as well as some late transition metals (copper, silver, gold) have also been observed, but only either at low temperatures in noble gas matrices, or in supersonic noble gas jets. Similar conditions were used to obtain the first few compounds of argon in 2000, such as argon fluorohydride (HArF), and some bound to the late transition metals copper, silver, and gold. As of 2007, no stable neutral molecules involving covalently bound helium or neon are known.
Extrapolation from periodic trends predict that oganesson should be the most reactive of the noble gases; more sophisticated theoretical treatments indicate greater reactivity than such extrapolations suggest, to the point where the applicability of the descriptor "noble gas" has been questioned. Oganesson is expected to be rather like silicon or tin in group 14: a reactive element with a common +4 and a less common +2 state, which at room temperature and pressure is not a gas but rather a solid semiconductor. Empirical / experimental testing will be required to validate these predictions. (On the other hand, flerovium, despite being in group 14, is predicted to be unusually volatile, which suggests noble gas-like properties.)
The noble gases—including helium—can form stable molecular ions in the gas phase. The simplest is the helium hydride molecular ion, HeH+, discovered in 1925. Because it is composed of the two most abundant elements in the universe, hydrogen and helium, it was believed to occur naturally in the interstellar medium, and it was finally detected in April 2019 using the airborne SOFIA telescope. In addition to these ions, there are many known neutral excimers of the noble gases. These are compounds such as ArF and KrF that are stable only when in an excited electronic state; some of them find application in excimer lasers.
In addition to the compounds where a noble gas atom is involved in a covalent bond, noble gases also form non-covalent compounds. The clathrates, first described in 1949, consist of a noble gas atom trapped within cavities of crystal lattices of certain organic and inorganic substances. The essential condition for their formation is that the guest (noble gas) atoms must be of appropriate size to fit in the cavities of the host crystal lattice. For instance, argon, krypton, and xenon form clathrates with hydroquinone, but helium and neon do not because they are too small or insufficiently polarizable to be retained. Neon, argon, krypton, and xenon also form clathrate hydrates, where the noble gas is trapped in ice.
 Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when , a spherical molecule consisting of 60
Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when , a spherical molecule consisting of 60 carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms, is exposed to noble gases at high pressure, complexes such as can be formed (the ''@'' notation indicates He is contained inside but not covalently bound to it). As of 2008, endohedral complexes with helium, neon, argon, krypton, and xenon have been created. These compounds have found use in the study of the structure and reactivity of fullerenes by means of the nuclear magnetic resonance of the noble gas atom.
Occurrence
The abundances of the noble gases in the universe decrease as their atomic numbers increase. Helium is the most common element in theuniverse
The universe is all of space and time and their contents. It comprises all of existence, any fundamental interaction, physical process and physical constant, and therefore all forms of matter and energy, and the structures they form, from s ...
after hydrogen, with a mass fraction of about 24%. Most of the helium in the universe was formed during Big Bang nucleosynthesis, but the amount of helium is steadily increasing due to the fusion of hydrogen in stellar nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
(and, to a very slight degree, the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of heavy elements).
Abundances on Earth follow different trends; for example, helium is only the third most abundant noble gas in the atmosphere. The reason is that there is no primordial helium in the atmosphere; due to the small mass of the atom, helium cannot be retained by the Earth's gravitational field
In physics, a gravitational field or gravitational acceleration field is a vector field used to explain the influences that a body extends into the space around itself. A gravitational field is used to explain gravitational phenomena, such as ...
. Helium on Earth comes from the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of heavy elements such as uranium and thorium found in the Earth's crust, and tends to accumulate in natural gas deposits. The abundance of argon, on the other hand, is increased as a result of the beta decay of potassium-40, also found in the Earth's crust, to form argon-40, which is the most abundant isotope of argon on Earth despite being relatively rare in the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
. This process is the basis for the potassium-argon dating method.
Xenon has an unexpectedly low abundance in the atmosphere, in what has been called the ''missing xenon problem''; one theory is that the missing xenon may be trapped in minerals inside the Earth's crust. Radon is formed in the lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
by the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
of radium. It can seep into buildings through cracks in their foundation and accumulate in areas that are not well ventilated. Due to its high radioactivity, radon presents a significant health hazard; it is implicated in an estimated 21,000 lung cancer deaths per year in the United States alone. Oganesson does not occur in nature and is instead created manually by scientists.
Extraction
Neon, argon, krypton, and xenon are obtained from air using the methods of liquefaction of gases, to convert elements to a liquid state, and fractional distillation, to separate mixtures into component parts. Helium is typically produced by separating it from natural gas, and radon is isolated from the radioactive decay of radium compounds. The prices of the noble gases are influenced by their natural abundance, with argon being the cheapest and xenon the most expensive. As an example, the adjacent table lists the 2004 prices in the United States for laboratory quantities of each gas.Biological chemistry
None of the elements in this group has any biological importance.Applications
solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
in fluids, especially in lipids
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins Vitamin A, A, Vitamin D, D, Vitamin E, E and Vitamin K, K), monoglycerides, diglycerides, phospholipids, and others. The fu ...
. Gases are absorbed by the blood and body tissues when under pressure like in scuba diving, which causes an anesthetic effect known as nitrogen narcosis. Due to its reduced solubility, little helium is taken into cell membranes, and when helium is used to replace part of the breathing mixtures, such as in trimix or heliox, a decrease in the narcotic effect of the gas at depth is obtained. Helium's reduced solubility offers further advantages for the condition known as decompression sickness
Decompression sickness (DCS; also called divers' disease, the bends, aerobullosis, and caisson disease) is a medical condition caused by dissolved gases emerging from Solution (chemistry), solution as bubbles inside the body tissues during D ...
, or ''the bends''. The reduced amount of dissolved gas in the body means that fewer gas bubbles form during the decrease in pressure of the ascent. Another noble gas, argon, is considered the best option for use as a drysuit inflation gas for scuba diving. Helium is also used as filling gas in nuclear fuel rods for nuclear reactors.
 Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a lifting gas in blimps and balloons: despite an 8.6% decrease in buoyancy compared to hydrogen, helium is not combustible.
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediates, by trapping them in an inert matrix at very low temperatures. Helium is used as the carrier medium in gas chromatography, as a filler gas for
Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a lifting gas in blimps and balloons: despite an 8.6% decrease in buoyancy compared to hydrogen, helium is not combustible.
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediates, by trapping them in an inert matrix at very low temperatures. Helium is used as the carrier medium in gas chromatography, as a filler gas for thermometers
A thermometer is a device that temperature measurement, measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperatur ...
, and in devices for measuring radiation, such as the Geiger counter and the bubble chamber. Helium and argon are both commonly used to shield welding arcs and the surrounding base metal from the atmosphere during welding and cutting, as well as in other metallurgical processes and in the production of silicon for the semiconductor industry.
 Noble gases are commonly used in
Noble gases are commonly used in lighting
Lighting or illumination is the deliberate use of light to achieve practical or aesthetic effects. Lighting includes the use of both artificial light sources like lamps and light fixtures, as well as natural illumination by capturing daylight. ...
because of their lack of chemical reactivity. Argon, mixed with nitrogen, is used as a filler gas for incandescent light bulbs. Krypton is used in high-performance light bulbs, which have higher color temperatures and greater efficiency, because it reduces the rate of evaporation of the filament more than argon; halogen lamps, in particular, use krypton mixed with small amounts of compounds of iodine or bromine. The noble gases glow in distinctive colors when used inside gas-discharge lamp
Gas-discharge lamps are a family of artificial light sources that generate light by sending an electric discharge through an ionization, ionized gas, a plasma (physics), plasma.
Typically, such lamps use a
noble gas (argon, neon, krypton, and x ...
s, such as " neon lights". These lights are called after neon but often contain other gases and phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or ...
s, which add various hues to the orange-red color of neon. Xenon is commonly used in xenon arc lamps, which, due to their nearly continuous spectrum that resembles daylight, find application in film projectors and as automobile headlamps.
The noble gases are used in excimer lasers, which are based on short-lived electronically excited molecules known as excimers. The excimers used for lasers may be noble gas dimers such as Ar2, Kr2 or Xe2, or more commonly, the noble gas is combined with a halogen in excimers such as ArF, KrF, XeF, or XeCl. These lasers produce ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
light, which, due to its short wavelength (193 nm for ArF and 248 nm for KrF), allows for high-precision imaging. Excimer lasers have many industrial, medical, and scientific applications. They are used for microlithography and microfabrication, which are essential for integrated circuit
An integrated circuit (IC), also known as a microchip or simply chip, is a set of electronic circuits, consisting of various electronic components (such as transistors, resistors, and capacitors) and their interconnections. These components a ...
manufacture, and for laser surgery
Laser surgery is a type of surgery that cuts tissue using a laser in contrast to using a scalpel.
Soft-tissue laser surgery is used in a variety of applications in humans ( general surgery, neurosurgery, ENT, dentistry, orthodontics, and ...
, including laser angioplasty and eye surgery.
Some noble gases have direct application in medicine. Helium is sometimes used to improve the ease of breathing of people with asthma. Xenon is used as an anesthetic because of its high solubility in lipids, which makes it more potent than the usual nitrous oxide, and because it is readily eliminated from the body, resulting in faster recovery. Xenon finds application in medical imaging of the lungs through hyperpolarized MRI. Radon, which is highly radioactive and is only available in minute amounts, is used in radiotherapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle ...
.
Noble gases, particularly xenon, are predominantly used in ion engines due to their inertness. Since ion engines are not driven by chemical reactions, chemically inert fuels are desired to prevent unwanted reaction between the fuel and anything else on the engine.
Oganesson is too unstable to work with and has no known application other than research.
Noble gases in Earth sciences application
The relative isotopic abundances of noble gases serve as an importantgeochemical
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the ...
tracing tool in earth science. They can unravel the Earth's degassing history and its effects to the surrounding environment (i.e., atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
composition). Due to their inert nature and low abundances, change in the noble gas concentration and their isotopic ratios can be used to resolve and quantify the processes influencing their current signatures across geological
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth s ...
settings.
Helium
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
has two abundant isotopes: helium-3, which is primordial with high abundance in earth's core and mantle, and helium-4, which originates from decay of radionuclides (232Th, 235,238U) abundant in the earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
. Isotopic ratios of helium are represented by RA value, a value relative to air measurement (3He/4He = 1.39*10−6). Volatiles that originate from the earth's crust have a 0.02-0.05 RA, which indicate an enrichment of helium-4. Volatiles that originate from deeper sources such as subcontinental lithospheric mantle (SCLM), have a 6.1± 0.9 RA and mid-oceanic ridge basalts (MORB) display higher values (8 ± 1 RA). Mantle plume samples have even higher values than > 8 RA. Solar wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy betwee ...
, which represent an unmodified primordial signature is reported to have ~ 330 RA.
Neon
Neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
has three main stable isotopes:20Ne, 21Ne and 22Ne, with 20Ne produced by cosmic nucleogenic reactions, causing high abundance in the atmosphere. 21Ne and 22Ne are produced in the earth's crust as a result of interactions between alpha and neutron particles with light elements; 18O, 19F and 24,25Mg. The neon ratios (20Ne/22Ne and 21Ne/22Ne) are systematically used to discern the heterogeneity in the Earth's mantle and volatile sources. Complimenting He isotope data, neon isotope data additionally provide insight to thermal evolution of Earth's systems.
Argon
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
has three stable isotopes: 36Ar, 38Ar and 40Ar. 36Ar and 38Ar are primordial, with their inventory on the earth's crust dependent on the equilibration of meteoric water with the crustal fluids. This explains huge inventory of 36Ar in the atmosphere. Production of these two isotopes (36Ar and 38Ar) is negligible within the earth's crust, only limited concentrations of 38Ar can be produced by interaction between alpha particles from decay of 235,238U and 232Th and light elements (37Cl and 41K). While 36Ar is continuously being produced by Beta-decay of 36Cl. 40Ar is a product of radiogenic decay of 40K. Different endmembers values for 40Ar/36Ar have been reported; Air = 295.5, MORB = 40,000, and crust = 3000.
Krypton
Krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is of ...
has several isotopes, with 78, 80, 82Kr being primordial, while 83,84, 86Kr results from spontaneous fission of 244Pu and radiogenic decay of 238U. Krypton's isotopes geochemical signature in mantle reservoirs resembling the modern atmosphere. preserves the solar-like primordial signature. Krypton isotopes have been used to decipher the mechanism of volatiles delivery to earth's system, which had great implication to evolution of earth (nitrogen, oxygen, and oxygen) and emergence of life. This is largely due to a clear distinction of krypton isotope signature from various sources such as chondritic material, solar wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy betwee ...
and comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
ary.
Xenon
Xenon has nine isotopes, most of which are produced by the radiogenic decay. Krypton and xenon noble gases requires pristine, robust geochemical sampling protocol to avoid atmospheric contamination. Furthermore, sophisticated instrumentation is required to resolve mass peaks among many isotopes with narrow mass difference during analysis.Sampling of noble gases
Noble gas measurements can be obtained from sources like volcanic vents, springs, and geothermal wells following specific sampling protocols. The classic specific sampling protocol include the following. * Copper tubes - These are standard refrigeration copper tubes, cut to ~10 cm³ with a 3/8” outer diameter, and are used for sampling volatile discharges by connecting an inverted funnel to the tube via TygonⓇ tubing, ensuring one-way inflow and preventing air contamination. Their malleability allows for cold welding or pinching off to seal the ends after sufficient flushing with the sample. ** Giggenbach bottles - Giggenbach bottles are evacuated glass flasks with a Teflon stopcock, used for sampling and storing gases. They require pre-evacuation before sampling, as noble gases accumulate in the headspace. These bottles were first invented and distributed by a Werner F. Giggenbach, a German chemist.
Giggenbach bottles - Giggenbach bottles are evacuated glass flasks with a Teflon stopcock, used for sampling and storing gases. They require pre-evacuation before sampling, as noble gases accumulate in the headspace. These bottles were first invented and distributed by a Werner F. Giggenbach, a German chemist.
= Analysis of noble gases
= Noble gases have numerous isotopes and subtle mass variation that requires high-precision detection systems. Originally, scientists used magnetic sector mass spectrometry, which is time-consuming and has low sensitivity due to "peak jumping mode". Multiple-collector mass spectrometers, like Quadrupole mass spectrometers (QMS), enable simultaneous detection of isotopes, improving sensitivity and throughput. Before analysis, sample preparation is essential due to the low abundance of noble gases, involving extraction, purification system. Extraction allows liberation of noble gases from their carrier (major phase; fluid or solid) while purification remove impurities and improve concentration per unit sample volume. Cryogenic traps are used for sequential analysis without peak interference by stepwise temperature raise. Research labs have successfully developed miniaturized field-based mass spectrometers, such as the portable mass spectrometerminiRuedi
, which can analyze noble gases with an analytical uncertainty of 1-3% using low-cost vacuum systems and quadrupole mass analyzers.

Discharge color
The color of gas discharge emission depends on several factors, including the following: * discharge parameters (local value of current density andelectric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
, temperature, etc. – note the color variation along the discharge in the top row);
* gas purity (even small fraction of certain gases can affect color);
* material of the discharge tube envelope – note suppression of the UV and blue components in the bottom-row tubes made of thick household glass.
See also
* Noble gas (data page), for extended tables of physical properties. * Noble metal, for metals that are resistant to corrosion or oxidation. * Inert gas, for any gas that is not reactive under normal circumstances. * Industrial gas * Octet ruleNotes
References
* * * * * * * * {{DEFAULTSORT:Noble Gas Groups (periodic table)