|
Hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843. In 2022, it was the 268th most commonly prescribed medication in the United States, with more than 900,000 prescriptions. Production Hydroquinone is produced industrially in two main ways.Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. . * The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Small amounts of catechol occur in fruits and vegetables. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) sublimated as a white efflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the chemical formula, formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated carbonyl compound, α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound. Preparation 1,4-Benzoquinone is prepared industrially by oxidation of hydroquinone, which can be obtained by several routes. One route involves oxidation of Diisopropylbenzenes, diisopropylbenzene and the Hock rearrangem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cumene Process
The cumene process (cumene-phenol process, Hock process) is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene (isopropyl benzene), the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944. This process converts two relatively cheap starting materials, benzene and propylene, into two more valuable ones, phenol and acetone. Other reactants required are oxygen from air and small amounts of a radical initiator. Most of the worldwide production of phenol and acetone is now based on this method. In 2022, nearly 10.8 million tonnes of phenol was produced by the cumene process. In order for this process to be economical, there must also be demand for the acetone by-product as well as the phenol. Steps of the process Cumene is formed in the gas-phase Friedel–Crafts alkylation of benzene by propene. Benzene and propene are compr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friedrich Wöhler
Friedrich Wöhler Royal Society of London, FRS(For) HonFRSE (; 31 July 180023 September 1882) was a German chemist known for his work in both organic chemistry, organic and inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the first to prepare several inorganic compounds, including silane and silicon nitride. Wöhler is also known for seminal contributions in organic chemistry, in particular, the Wöhler synthesis of urea. His synthesis of the organic compound urea in the laboratory from inorganic substances contradicted the belief that organic compounds could only be produced by living organisms due to a "life force". However, the exact extent of Wöhler's role in diminishing the belief in vitalism is considered by some to be questionable. Biography Friedrich Wöhler was born in Eschersheim, Germany, and was the son of a veterinarian. As a boy, he showed interest in mineral collecting, drawing, and science. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenediol
In organic chemistry, dihydroxybenzenes (benzenediols) are organic compounds in which two hydroxyl groups () are Electrophilic aromatic substitution, substituted onto a benzene ring (). These aromatic compounds are classed as phenols. There are three structural isomers: 1,2-dihydroxybenzene (the ''ortho'' isomer) is commonly known as catechol, 1,3-dihydroxybenzene (the ''meta'' isomer) is commonly known as resorcinol, and 1,4-dihydroxybenzene (the ''para'' isomer) is commonly known as hydroquinone. : All three of these compounds are colorless to white granular solids at room temperature and pressure, but upon exposure to oxygen they may darken. All three isomers have the chemical formula . Similar to other phenols, the hydroxyl groups on the aromatic ring of a benzenediol are weakly acidic. Each benzenediol can lose an from one of the hydroxyls to form a type of phenolate ion. The Dakin oxidation is an organic redox reaction in which an ''ortho''- or ''para''-hydroxylated phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resorcinol
Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or ''meta- (chemistry), meta''-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide. Production Resorcinol is produced in several steps from benzene, starting with dialkylation with propylene to give 1,3-Diisopropylbenzene, 1,3-diisopropylbenzene. Oxidation and Hock rearrangement of this disubstituted arene gives acetone and resorcinol. Resorcinol is a relatively inexpensive chemical. It is produced in only a very few locations around the world (as of 2010 only four commercial plants were known to be operative: in the United States, Germany, China, and Japan), and is the determining factor in the cost of Phenol formaldehyde resin, PRF adhesives. Production in the United States ended in 2017 with the cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
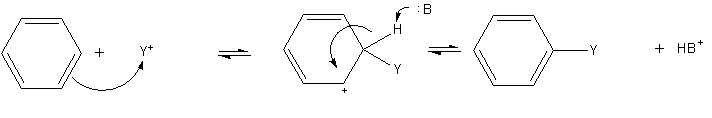
Benzene Ring
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound. Benzene is classified as a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility. Hydroxylation of a hydrocarbon is an oxidation, thus a step in degradation. Biological hydroxylation In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. These enzymes insert an O atom into a bond. Typical stoichiometries for the hydroxylation of a generic hydrocarbon are these: : : Since itself is a slow and unselective hydroxylating agent, catalysts are required to accelerate the pace of the process and to introduce selectivity. Hydroxylation is often the first step in the degradation of organic compounds in air. Hydroxylation is important in detoxification since it converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly into water and elemental oxygen when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a Stabilizer (chemistry), stabilizer in a weakly acidic solution in an opaque bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |