calcium carbonate on:
[Wikipedia]
[Google]
[Amazon]
 Calcium carbonate is a
Calcium carbonate is a

 Carbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. Calcium carbonate occurs as
Carbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. Calcium carbonate occurs as  In warm, clear tropical waters
In warm, clear tropical waters
 Calcium carbonate is widely used medicinally as an inexpensive dietary calcium supplement or gastric antacid (such as Tums and Eno). It may be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with
Calcium carbonate is widely used medicinally as an inexpensive dietary calcium supplement or gastric antacid (such as Tums and Eno). It may be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with
 Calcium carbonate is poorly soluble in pure water (47 mg/L at normal atmospheric partial pressure as shown below).
The equilibrium of its solution is given by the equation (with dissolved calcium carbonate on the right):
:
where the solubility product for is given as anywhere from ''K''sp = to ''K''sp = at 25 °C, depending upon the data source. What the equation means is that the product of molar concentration of calcium ions ( moles of dissolved per liter of solution) with the molar concentration of dissolved cannot exceed the value of ''K''sp. This seemingly simple solubility equation, however, must be taken along with the more complicated equilibrium of
Calcium carbonate is poorly soluble in pure water (47 mg/L at normal atmospheric partial pressure as shown below).
The equilibrium of its solution is given by the equation (with dissolved calcium carbonate on the right):
:
where the solubility product for is given as anywhere from ''K''sp = to ''K''sp = at 25 °C, depending upon the data source. What the equation means is that the product of molar concentration of calcium ions ( moles of dissolved per liter of solution) with the molar concentration of dissolved cannot exceed the value of ''K''sp. This seemingly simple solubility equation, however, must be taken along with the more complicated equilibrium of
 In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of
In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of
The British Calcium Carbonate Association – What is calcium carbonate
{{Authority control Calcium compounds Carbonates Limestone Phosphate binders Excipients Antacids Food stabilizers E-number additives
 Calcium carbonate is a
Calcium carbonate is a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. It is a common substance found in rocks as the mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
and aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
, most notably in chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Ch ...
and limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, eggshell
An eggshell is the outer covering of a hard-shelled egg (biology), egg and of some forms of eggs with soft outer coats.
Worm eggs
Nematode eggs present a two layered structure: an external vitellin layer made of chitin that confers mechanical ...
s, gastropod shell
The gastropod shell is part of the body of many gastropods, including snails, a kind of mollusc. The shell is an exoskeleton, which protects from predators, mechanical damage, and dehydration, but also serves for muscle attachment and calcium ...
s, shellfish
Shellfish, in colloquial and fisheries usage, are exoskeleton-bearing Aquatic animal, aquatic invertebrates used as Human food, food, including various species of Mollusca, molluscs, crustaceans, and echinoderms. Although most kinds of shellfish ...
skeletons and pearl
A pearl is a hard, glistening object produced within the soft tissue (specifically the mantle (mollusc), mantle) of a living Exoskeleton, shelled mollusk or another animal, such as fossil conulariids. Just like the shell of a mollusk, a pear ...
s. Materials containing much calcium carbonate or resembling it are described as calcareous
Calcareous () is an adjective meaning "mostly or partly composed of calcium carbonate", in other words, containing lime (mineral), lime or being chalky. The term is used in a wide variety of Science, scientific disciplines.
In zoology
''Calcare ...
. Calcium carbonate is the active ingredient in agricultural lime
Agricultural lime, also called aglime, agricultural limestone, garden lime or liming, is a soil additive made from pulverized limestone or chalk. The primary active component is calcium carbonate. Additional chemicals vary depending on the mineral ...
and is produced when calcium ions in hard water react with carbonate ions to form limescale
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially that for hot water. It is also often found as a similar deposit on the inner surfaces of old ...
. It has medical use as a calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
supplement or as an antacid
An antacid is a substance which neutralization (chemistry), neutralizes gastric acid, stomach acidity and is used to relieve heartburn, indigestion, or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhe ...
, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.
Chemistry
Calcium carbonate shares the typical properties of othercarbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s. Notably, it:
*reacts with acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s, releasing carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
which quickly disintegrates into carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
:
:
*releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally f ...
(to above 840 °C in the case of ), to form calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
, CaO, commonly called quicklime
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containin ...
, with reaction enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
178 kJ/mol:
:
* reacts with gaseous hydrogen to form methane and water vapor plus solid calcium oxide or calcium hydroxide depending on temperature and product gas composition. Various metals including palladium and nickel are catalysts for the reaction.
Calcium carbonate reacts with water that is saturated with carbon dioxide to form the soluble calcium bicarbonate.
:
This reaction is important in the erosion
Erosion is the action of surface processes (such as Surface runoff, water flow or wind) that removes soil, Rock (geology), rock, or dissolved material from one location on the Earth's crust#Crust, Earth's crust and then sediment transport, tran ...
of carbonate rock
Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3), and Dolomite (rock), dolomite rock (also kn ...
, forming caverns, and leads to hard water in many regions.
An unusual form of calcium carbonate is the hexahydrate ikaite, . Ikaite is stable only below 8 °C.
Preparation
The vast majority of calcium carbonate used in industry is extracted by mining or quarrying. Pure calcium carbonate (such as for food or pharmaceutical use), can be produced from a pure quarried source (usuallymarble
Marble is a metamorphic rock consisting of carbonate minerals (most commonly calcite (CaCO3) or Dolomite (mineral), dolomite (CaMg(CO3)2) that have recrystallized under the influence of heat and pressure. It has a crystalline texture, and is ty ...
).
Alternatively, calcium carbonate is prepared from calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
. Water is added to give calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
then carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
is passed through this solution to precipitate
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemic ...
the desired calcium carbonate, referred to in the industry as precipitated calcium carbonate (PCC). This process is called carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although thi ...
:
:
:
In a laboratory, calcium carbonate can easily be crystallized from calcium chloride
Calcium chloride is an inorganic compound, a Salt (chemistry), salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with cal ...
(), by placing an aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
of in a desiccator
Desiccators are sealable enclosures containing desiccants used for preserving moisture-sensitive items such as cobalt chloride paper for another use. A common use for desiccators is to protect chemicals which are hygroscopic or which react wit ...
alongside ammonium carbonate . In the desiccator, ammonium carbonate is exposed to air and decomposes into ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, carbon dioxide, and water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
. The carbon dioxide then diffuses into the aqueous solution of calcium chloride, reacts with the calcium ions and the water, and forms calcium carbonate.
Structure
The thermodynamically stable form of under normal conditions ishexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
β- (the mineral calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
). Other forms can be prepared, the denser (2.83 g/cm3) orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
λ- (the mineral aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
) and hexagonal μ-, occurring as the mineral vaterite
Vaterite is a mineral, a polymorphism (materials science), polymorph of calcium carbonate (calcium, Cacarbon, Coxygen, O3). It was named after the German mineralogist Heinrich Vater. It is also known as mu-calcium carbonate (μ-CaCO3). Vaterite b ...
. The aragonite form can be prepared by precipitation at temperatures above 85 °C; the vaterite form can be prepared by precipitation at 60 °C. Calcite contains calcium atoms coordinated by six oxygen atoms; in aragonite they are coordinated by nine oxygen atoms. The vaterite structure is not fully understood. Magnesium carbonate () has the calcite structure, whereas strontium carbonate
Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite.
Chemical properties
Strontium carbonate is a white, odorless, tasteless powder ...
() and barium carbonate
Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of ...
() adopt the aragonite structure, reflecting their larger ionic radii.
Polymorphs
Calcium carbonate crystallizes in threeanhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
polymorphs, of which calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
is the thermodynamically most stable at room temperature, aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
is only slightly less so, and vaterite
Vaterite is a mineral, a polymorphism (materials science), polymorph of calcium carbonate (calcium, Cacarbon, Coxygen, O3). It was named after the German mineralogist Heinrich Vater. It is also known as mu-calcium carbonate (μ-CaCO3). Vaterite b ...
is the least stable.
Crystal structure
Thecalcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
crystal structure is trigonal
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the tr ...
, with space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
Rc (No. 167 in the International Tables for Crystallography), and Pearson symbol hR10. Aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
is orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
, with space group Pmcn (No 62), and Pearson Symbol oP20. Vaterite
Vaterite is a mineral, a polymorphism (materials science), polymorph of calcium carbonate (calcium, Cacarbon, Coxygen, O3). It was named after the German mineralogist Heinrich Vater. It is also known as mu-calcium carbonate (μ-CaCO3). Vaterite b ...
is composed of at least two different coexisting crystallographic structures. The major structure exhibits hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
symmetry in space group P63/mmc, the minor structure is still unknown.
Crystallization
All three polymorphs crystallize simultaneously from aqueous solutions under ambient conditions. In additive-free aqueous solutions, calcite forms easily as the major product, while aragonite appears only as a minor product. At high saturation, vaterite is typically the first phase precipitated, which is followed by a transformation of the vaterite to calcite. This behavior seems to followOstwald's rule
In materials science, Ostwald's rule or Ostwald's step rule, conceived by Wilhelm Ostwald, describes the formation of Polymorphism (materials science), polymorphs. The rule states that usually the less Chemical stability, stable polymorph crystalli ...
, in which the least stable polymorph crystallizes first, followed by the crystallization of different polymorphs via a sequence of increasingly stable phases. However, aragonite, whose stability lies between those of vaterite and calcite, seems to be the exception to this rule, as aragonite does not form as a precursor to calcite under ambient conditions.
Aragonite occurs in majority when the reaction conditions inhibit the formation of calcite and/or promote the nucleation of aragonite. For example, the formation of aragonite is promoted by the presence of magnesium ions, or by using proteins and peptides derived from biological calcium carbonate. Some polyamines such as cadaverine
Cadaverine is an organic compound with the formula (CH2)5(NH2)2. Classified as a diamine, it is a colorless liquid with an unpleasant odor. It is present in small quantities in living organisms but is often associated with the putrefaction of Tiss ...
and Poly(ethylene imine)
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon Aliphatic_compound, aliphatic ''CHCH'' spacers. Linear polyethyleneimines contain all Amines#Classification_of_amines, secondary ...
have been shown to facilitate the formation of aragonite over calcite. Solid‑state NMR analysis has revealed that poly‑aspartate-stabilized ACC contains water molecules that undergo millisecond-timescale flips, illustrating dynamic hydration as a key factor in delaying crystallization.
Selection by organisms
Organisms, such asmolluscs
Mollusca is a phylum of protostome, protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant taxon, extant species of molluscs are recognized, making it the second-largest animal phylum ...
and arthropod
Arthropods ( ) are invertebrates in the phylum Arthropoda. They possess an arthropod exoskeleton, exoskeleton with a cuticle made of chitin, often Mineralization (biology), mineralised with calcium carbonate, a body with differentiated (Metam ...
s, have shown the ability to grow all three crystal polymorphs of calcium carbonate, mainly as protection (shells) and muscle attachments. Moreover, they exhibit a remarkable capability of phase selection over calcite and aragonite, and some organisms can switch between the two polymorphs. The ability of phase selection is usually attributed to the use of specific macromolecules or combinations of macromolecules by such organisms.
Occurrence

Geological sources
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
and vaterite
Vaterite is a mineral, a polymorphism (materials science), polymorph of calcium carbonate (calcium, Cacarbon, Coxygen, O3). It was named after the German mineralogist Heinrich Vater. It is also known as mu-calcium carbonate (μ-CaCO3). Vaterite b ...
are pure calcium carbonate minerals. Industrially important source rocks which are predominantly calcium carbonate include limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Ch ...
, marble
Marble is a metamorphic rock consisting of carbonate minerals (most commonly calcite (CaCO3) or Dolomite (mineral), dolomite (CaMg(CO3)2) that have recrystallized under the influence of heat and pressure. It has a crystalline texture, and is ty ...
and travertine.
Biological sources
Eggshell
An eggshell is the outer covering of a hard-shelled egg (biology), egg and of some forms of eggs with soft outer coats.
Worm eggs
Nematode eggs present a two layered structure: an external vitellin layer made of chitin that confers mechanical ...
s, snail
A snail is a shelled gastropod. The name is most often applied to land snails, terrestrial molluscs, terrestrial pulmonate gastropod molluscs. However, the common name ''snail'' is also used for most of the members of the molluscan class Gas ...
shells and most seashell
A seashell or sea shell, also known simply as a shell, is a hard, protective outer layer usually created by an animal or organism that lives in the sea. Most seashells are made by Mollusca, mollusks, such as snails, clams, and oysters ...
s are predominantly calcium carbonate and can be used as industrial sources of that chemical. Oyster
Oyster is the common name for a number of different families of salt-water bivalve molluscs that live in marine or brackish habitats. In some species, the valves are highly calcified, and many are somewhat irregular in shape. Many, but no ...
shells have enjoyed recent recognition as a source of dietary calcium, but are also a practical industrial source. Dark green vegetables such as broccoli
Broccoli (''Brassica oleracea'' var. ''italica'') is an edible green plant in the Brassicaceae, cabbage family (family Brassicaceae, genus ''Brassica'') whose large Pseudanthium, flowering head, plant stem, stalk and small associated leafy gre ...
and kale
Kale (), also called leaf cabbage, belongs to a group of cabbage (''Brassica oleracea'') cultivars primarily grown for their Leaf vegetable, edible leaves; it has also been used as an ornamental plant. Its multiple different cultivars vary quite ...
contain dietarily significant amounts of calcium carbonate, but they are not practical as an industrial source.
Annelid
The annelids (), also known as the segmented worms, are animals that comprise the phylum Annelida (; ). The phylum contains over 22,000 extant species, including ragworms, earthworms, and leeches. The species exist in and have adapted to vario ...
s in the family Lumbricidae, earthworms, possess a regionalization of the digestive track called calciferous glands, Kalkdrüsen, or glandes de Morren, that processes calcium and into calcium carbonate, which is later excreted into the dirt. The function of these glands is unknown but is believed to serve as a regulation mechanism within the animals' tissues. This process is ecologically significant, stabilizing the pH of acid soils.
Extraterrestrial
Beyond Earth, strong evidence suggests the presence of calcium carbonate onMars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
. Signs of calcium carbonate have been detected at more than one location (notably at Gusev and Huygens craters). This provides some evidence for the past presence of liquid water.
Geology
 Carbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. Calcium carbonate occurs as
Carbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. Calcium carbonate occurs as aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
, calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
and dolomite as significant constituents of the calcium cycle. The carbonate mineral
Carbonate minerals are those minerals containing the carbonate ion, .
Carbonate divisions Anhydrous carbonates
*Calcite group: trigonal
**Calcite CaCO3
**Gaspéite (Ni,Mg,Fe2+)CO3
**Magnesite MgCO3
**Otavite CdCO3
**Rhodochrosite MnCO3
**Sider ...
s form the rock types: limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Ch ...
, marble
Marble is a metamorphic rock consisting of carbonate minerals (most commonly calcite (CaCO3) or Dolomite (mineral), dolomite (CaMg(CO3)2) that have recrystallized under the influence of heat and pressure. It has a crystalline texture, and is ty ...
, travertine, tufa
Tufa is a variety of limestone formed when carbonate minerals precipitation (chemistry), precipitate out of water in ambient temperature, unheated rivers or lakes. hot spring, Geothermally heated hot springs sometimes produce similar (but less ...
, and others.
 In warm, clear tropical waters
In warm, clear tropical waters coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s are more abundant than towards the poles where the waters are cold. Calcium carbonate contributors, including plankton
Plankton are the diverse collection of organisms that drift in Hydrosphere, water (or atmosphere, air) but are unable to actively propel themselves against ocean current, currents (or wind). The individual organisms constituting plankton are ca ...
(such as coccolith
Coccoliths are individual plates or scales of calcium carbonate formed by coccolithophores (single-celled phytoplankton such as ''Emiliania huxleyi'') and cover the cell surface arranged in the form of a spherical shell, called a '' coccosphere'' ...
s and planktic foraminifera
Foraminifera ( ; Latin for "hole bearers"; informally called "forams") are unicellular organism, single-celled organisms, members of a phylum or class (biology), class of Rhizarian protists characterized by streaming granular Ectoplasm (cell bio ...
), coralline algae, sponges
Sponges or sea sponges are primarily marine invertebrates of the animal phylum Porifera (; meaning 'pore bearer'), a basal clade and a sister taxon of the diploblasts. They are sessile filter feeders that are bound to the seabed, and ar ...
, brachiopod
Brachiopods (), phylum (biology), phylum Brachiopoda, are a phylum of animals that have hard "valves" (shells) on the upper and lower surfaces, unlike the left and right arrangement in bivalve molluscs. Brachiopod valves are hinged at the rear e ...
s, echinoderm
An echinoderm () is any animal of the phylum Echinodermata (), which includes starfish, brittle stars, sea urchins, sand dollars and sea cucumbers, as well as the sessile sea lilies or "stone lilies". While bilaterally symmetrical as ...
s, bryozoa
Bryozoa (also known as the Polyzoa, Ectoprocta or commonly as moss animals) are a phylum of simple, aquatic animal, aquatic invertebrate animals, nearly all living in sedentary Colony (biology), colonies. Typically about long, they have a spe ...
and mollusks, are typically found in shallow water environments where sunlight and filterable food are more abundant. Cold-water carbonates do exist at higher latitudes but have a very slow growth rate. The calcification
Calcification is the accumulation of calcium salts in a body tissue. It normally occurs in the formation of bone, but calcium can be deposited abnormally in soft tissue,Miller, J. D. Cardiovascular calcification: Orbicular origins. ''Nature M ...
processes are changed by ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
.
Where the oceanic crust
Oceanic crust is the uppermost layer of the oceanic portion of the tectonic plates. It is composed of the upper oceanic crust, with pillow lavas and a dike complex, and the lower oceanic crust, composed of troctolite, gabbro and ultramaf ...
is subducted under a continental plate sediments will be carried down to warmer zones in the asthenosphere
The asthenosphere () is the mechanically weak and ductile region of the upper mantle of Earth. It lies below the lithosphere, at a depth between c. below the surface, and extends as deep as . However, the lower boundary of the asthenosphere i ...
and lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
. Under these conditions calcium carbonate decomposes to produce carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
which, along with other gases, give rise to explosive volcanic eruptions.
Carbonate compensation depth
Thecarbonate compensation depth
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate pa ...
(CCD) is the point in the ocean where the rate of precipitation of calcium carbonate is balanced by the rate of dissolution due to the conditions present. Deep in the ocean, the temperature drops and pressure increases. Increasing pressure also increases the solubility of calcium carbonate. Calcium carbonate is unusual in that its solubility increases with decreasing temperature. The carbonate compensation depth ranges from 4,000 to 6,000 meters below sea level in modern oceans, and the various polymorphs (calcite, aragonite) have different compensation depths based on their stability.
Role in taphonomy
Calcium carbonate can preserve fossils throughpermineralization
Permineralization is a process of fossilization of bones and tissues in which mineral deposits form internal Casting, casts of organisms. Carried by water, these minerals fill the spaces within organic tissue. Because of the nature of the casts, p ...
. Most of the vertebrate fossils of the Two Medicine Formation—a geologic formation known for its duck-billed dinosaur eggs—are preserved by permineralization. This type of preservation conserves high levels of detail, even down to the microscopic level. However, it also leaves specimens vulnerable to weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
when exposed to the surface.
Trilobite
Trilobites (; meaning "three-lobed entities") are extinction, extinct marine arthropods that form the class (biology), class Trilobita. One of the earliest groups of arthropods to appear in the fossil record, trilobites were among the most succ ...
populations were once thought to have composed the majority of aquatic life during the Cambrian
The Cambrian ( ) is the first geological period of the Paleozoic Era, and the Phanerozoic Eon. The Cambrian lasted 51.95 million years from the end of the preceding Ediacaran period 538.8 Ma (million years ago) to the beginning of the Ordov ...
, due to the fact that their calcium carbonate-rich shells were more easily preserved than those of other species, which had purely chitin
Chitin (carbon, C8hydrogen, H13oxygen, O5nitrogen, N)n ( ) is a long-chain polymer of N-Acetylglucosamine, ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is the second most abundant polysaccharide in nature (behind only cell ...
ous shells.
Uses
Construction
The main use of calcium carbonate is in the construction industry, either as a building material, or limestone aggregate for road building, as an ingredient ofcement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mi ...
, or as the starting material for the preparation of builders' lime by burning in a kiln
A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or Chemical Changes, chemical changes. Kilns have been used for millennia to turn objects m ...
. However, because of weathering mainly caused by acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
, calcium carbonate (in limestone form) is no longer used for building purposes on its own, but only as a raw primary substance for building materials.
Calcium carbonate is also used in the purification of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
from iron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the f ...
in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being supplied above atmospheric pressure.
In a ...
. The carbonate is calcined ''in situ'' to give calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
, which forms a slag
The general term slag may be a by-product or co-product of smelting (pyrometallurgical) ores and recycled metals depending on the type of material being produced. Slag is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be c ...
with various impurities present, and separates from the purified iron.
In the oil industry
The petroleum industry, also known as the oil industry, includes the global processes of exploration, extraction, refining, transportation (often by oil tankers and pipelines), and marketing of petroleum products. The largest volume products ...
, calcium carbonate is added to drilling fluid
In geotechnical engineering, drilling fluid, also known as drilling mud, is used to aid the drilling of boreholes into the earth. Used while drilling oil and natural gas wells and on exploration drilling rigs, drilling fluids are also use ...
s as a formation-bridging and filtercake-sealing agent; it is also a weighting material which increases the density of drilling fluids to control the downhole pressure. Calcium carbonate is added to swimming pool
A swimming pool, swimming bath, wading pool, paddling pool, or simply pool, is a structure designed to hold water to enable Human swimming, swimming and associated activities. Pools can be built into the ground (in-ground pools) or built abo ...
s, as a pH corrector for maintaining alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer s ...
and offsetting the acidic properties of the disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than ...
agent.
It is also used as a raw material in the refining of sugar from sugar beet
A sugar beet is a plant whose root contains a high concentration of sucrose and that is grown commercially for sugar production. In plant breeding, it is known as the Altissima cultivar group of the common beet (''Beta vulgaris''). Together with ...
; it is calcined in a kiln with anthracite
Anthracite, also known as hard coal and black coal, is a hard, compact variety of coal that has a lustre (mineralogy)#Submetallic lustre, submetallic lustre. It has the highest carbon content, the fewest impurities, and the highest energy densit ...
to produce calcium oxide and carbon dioxide. This burnt lime is then slaked in fresh water to produce a calcium hydroxide suspension for the precipitation of impurities in raw juice during carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although thi ...
.
Calcium carbonate in the form of chalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Ch ...
has traditionally been a major component of blackboard
A blackboard or a chalkboard is a reusable writing surface on which text or drawings are made with sticks of calcium sulphate or calcium carbonate, better known as chalk.
Blackboards were originally made of smooth, thin sheets of black or da ...
chalk. However, modern manufactured chalk is mostly gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
, hydrated calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
. Calcium carbonate is a main source for growing biorock. Precipitated calcium carbonate (PCC), pre-dispersed in slurry
A slurry is a mixture of denser solids suspended in liquid, usually water. The most common use of slurry is as a means of transporting solids or separating minerals, the liquid being a carrier that is pumped on a device such as a centrifugal pu ...
form, is a common filler material for latex gloves with the aim of achieving maximum saving in material and production costs.
Fine ground calcium carbonate (GCC) is an essential ingredient in the microporous film used in diapers
A diaper (, North American English) or a nappy (British English, Australian English, Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or containing waste products to ...
and some building films, as the pores are nucleated around the calcium carbonate particles during the manufacture of the film by biaxial stretching. GCC and PCC are used as a filler in paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
because they are cheaper than wood fiber
Wood fibres (also spelled wood fibers, see spelling differences) are usually cellulosic elements that are extracted from trees and used to make materials including paper.
The end paper product (paper, paperboard, tissue, cardboard, etc.) dictate ...
. Printing and writing paper can contain 10–20% calcium carbonate. In North America, calcium carbonate has begun to replace kaolin
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina (). ...
in the production of glossy paper. Europe has been practicing this as alkaline papermaking
Papermaking is the manufacture of paper and cardboard, which are used widely for printing, writing, and packaging, among many other purposes. Today almost all paper is Pulp and paper industry, made using industrial machinery, while handmade pape ...
or acid-free papermaking for some decades. PCC used for paper filling and paper coatings is precipitated and prepared in a variety of shapes and sizes having characteristic narrow particle size distributions and equivalent spherical diameters of 0.4 to 3 micrometers.
Calcium carbonate is widely used as an extender in paint
Paint is a material or mixture that, when applied to a solid material and allowed to dry, adds a film-like layer. As art, this is used to create an image or images known as a painting. Paint can be made in many colors and types. Most paints are ...
s, in particular matte emulsion paint where typically 30% by weight of the paint is either chalk or marble. It is also a popular filler in plastics. Some typical examples include around 15–20% loading of chalk in unplasticized polyvinyl chloride (uPVC) drainpipes, 5–15% loading of stearate-coated chalk or marble in uPVC window profile. PVC cables can use calcium carbonate at loadings of up to 70 phr (parts per hundred parts of resin) to improve mechanical properties (tensile strength and elongation) and electrical properties (volume resistivity). Polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
compounds are often filled with calcium carbonate to increase rigidity, a requirement that becomes important at high usage temperatures. Here the percentage is often 20–40%. It also routinely used as a filler in thermosetting resins (sheet and bulk molding compounds) and has also been mixed with ABS, and other ingredients, to form some types of compression molded "clay" poker chips. Precipitated calcium carbonate, made by dropping calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
into water, is used by itself or with additives as a white paint, known as whitewash
Whitewash, calcimine, kalsomine, calsomine, asbestis or lime paint is a type of paint made from slaked lime ( calcium hydroxide, Ca(OH)2) or chalk (calcium carbonate, CaCO3), sometimes known as "whiting". Various other additives are sometimes ...
ing.
Calcium carbonate is added to a wide range of trade and do it yourself
"Do it yourself" ("DIY") is the method of building, wikt:modification, modifying, or repairing things by oneself without the direct aid of professionals or certified experts. Academic research has described DIY as behaviors where "individuals ...
adhesives, sealants, and decorating fillers. Ceramic tile adhesives typically contain 70% to 80% limestone. Decorating crack fillers contain similar levels of marble or dolomite. It is also mixed with putty in setting stained glass
Stained glass refers to coloured glass as a material or art and architectural works created from it. Although it is traditionally made in flat panels and used as windows, the creations of modern stained glass artists also include three-dimensio ...
windows, and as a resist to prevent glass from sticking to kiln shelves when firing glazes and paints at high temperature.
In ceramic glaze applications, calcium carbonate is known as ''whiting'', and is a common ingredient for many glazes in its white powdered form. When a glaze containing this material is fired in a kiln, the whiting acts as a flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications in physics. For transport phe ...
material in the glaze. Ground calcium carbonate is an abrasive (both as scouring powder and as an ingredient of household scouring creams), in particular in its calcite form, which has the relatively low hardness level of 3 on the Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
, and will therefore not scratch glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
and most other ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
s, enamel, bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloid ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, and steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
, and have a moderate effect on softer metals like aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
and copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
. A paste made from calcium carbonate and deionized water can be used to clean tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
on silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
.
Health and diet
 Calcium carbonate is widely used medicinally as an inexpensive dietary calcium supplement or gastric antacid (such as Tums and Eno). It may be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with
Calcium carbonate is widely used medicinally as an inexpensive dietary calcium supplement or gastric antacid (such as Tums and Eno). It may be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with chronic kidney failure
Chronic kidney disease (CKD) is a type of long-term kidney disease, defined by the sustained presence of abnormal kidney function and/or abnormal kidney structure. To meet criteria for CKD, the abnormalities must be present for at least three mo ...
). It is used in the pharmaceutical industry as an inert filler for tablets and other pharmaceuticals
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the ...
.
Calcium carbonate is used in the production of calcium oxide as well as toothpaste and has seen a resurgence as a food preservative and color retainer, when used in or with products such as organic apples.
Calcium carbonate is used therapeutically as phosphate binder in patients on maintenance haemodialysis. It is the most common form of phosphate binder prescribed, particularly in non-dialysis chronic kidney disease. Calcium carbonate is the most commonly used phosphate binder, but clinicians are increasingly prescribing the more expensive, non-calcium-based phosphate binders, particularly sevelamer.
Excess calcium from supplements, fortified food, and high-calcium diets can cause milk-alkali syndrome
Milk-alkali syndrome (MAS), also referred to as calcium-alkali syndrome, is the third most common cause of elevated blood calcium levels ( hypercalcemia). Milk-alkali syndrome is characterized by hypercalcemia, metabolic alkalosis, and acute ki ...
, which has serious toxicity and can be fatal. In 1915, Bertram Sippy introduced the "Sippy regimen" of hourly ingestion of milk and cream, and the gradual addition of eggs and cooked cereal, for 10 days, combined with alkaline powders, which provided symptomatic relief for peptic ulcer disease. Over the next several decades, the Sippy regimen resulted in kidney failure
Kidney failure, also known as renal failure or end-stage renal disease (ESRD), is a medical condition in which the kidneys can no longer adequately filter waste products from the blood, functioning at less than 15% of normal levels. Kidney fa ...
, alkalosis
Alkalosis is the result of a process reducing hydrogen ion concentration of arterial blood plasma (alkalemia). In contrast to acidemia (serum pH 7.35 or lower), alkalemia occurs when the serum pH is higher than normal (7.45 or higher). Alkalosis ...
, and hypercalcaemia, mostly in men with peptic ulcer disease. These adverse effects were reversed when the regimen stopped, but it was fatal in some patients with protracted vomiting. Milk-alkali syndrome declined in men after effective treatments for peptic ulcer
Peptic ulcer disease is when the inner part of the stomach's gastric mucosa (lining of the stomach), the first part of the small intestine, or sometimes the lower esophagus, gets damaged. An ulcer in the stomach is called a gastric ulcer, while ...
disease arose. Since the 1990s it has been most frequently reported in women taking calcium supplements above the recommended range of 1.2 to 1.5 grams daily, for prevention and treatment of osteoporosis, and is exacerbated by dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
. Calcium has been added to over-the-counter products, which contributes to inadvertent excessive intake. Excessive calcium intake can lead to hypercalcemia, complications of which include vomiting, abdominal pain and altered mental status.
As a food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
it is designated E170, and it has an INS number of 170. Used as an acidity regulator
Acidity regulators, or pH control agents, are food additives used to change or maintain pH ( acidity or basicity). They can be organic or mineral acids, bases, neutralizing agents, or buffering agents. Typical agents include the following ac ...
, anticaking agent
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps ( caking) and for easing packaging, transport, flowability, and consumption. Caking mechanism ...
, stabilizer or color
Color (or colour in English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is the visual perception based on the electromagnetic spectrum. Though co ...
it is approved for usage in the EU, US and Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
and New Zealand
New Zealand () is an island country in the southwestern Pacific Ocean. It consists of two main landmasses—the North Island () and the South Island ()—and List of islands of New Zealand, over 600 smaller islands. It is the List of isla ...
. It is "added by law to all UK milled bread flour except wholemeal". It is used in some soy milk
Soy milk (or soymilk), also known as soya milk, is a plant-based milk produced by soaking and grinding soybeans, boiling the mixture, and filtering out remaining particulates. It is a stable emulsion of oil, water, and protein. Its original ...
and almond milk
Almond milk is a plant-based milk substitute with a watery texture and nutty flavor manufactured from almonds, although some types or brands are flavored in imitation of cow's milk. It does not contain cholesterol or lactose and is low in saturat ...
products as a source of dietary calcium; at least one study suggests that calcium carbonate might be as bioavailable as the calcium in cow's milk
Milk is a white liquid food produced by the mammary glands of lactating mammals. It is the primary source of nutrition for young mammals (including breastfed human infants) before they are able to digest solid food. Milk contains many nutr ...
. Calcium carbonate is also used as a firming agent in many canned and bottled vegetable products.
Several calcium supplement formulations have been documented to contain the chemical element lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
, posing a public health
Public health is "the science and art of preventing disease, prolonging life and promoting health through the organized efforts and informed choices of society, organizations, public and private, communities and individuals". Analyzing the de ...
concern. Lead is commonly found in natural sources of calcium.
Agriculture and aquaculture
Agricultural lime
Agricultural lime, also called aglime, agricultural limestone, garden lime or liming, is a soil additive made from pulverized limestone or chalk. The primary active component is calcium carbonate. Additional chemicals vary depending on the mineral ...
, powdered chalk or limestone, is used as a cheap method of neutralising acidic soil
Soil pH is a measure of the acidity or basicity (alkalinity) of a soil. Soil pH is a key characteristic that can be used to make informative analysis both qualitative and quantitatively regarding soil characteristics. pH is defined as the nega ...
, making it suitable for planting, also used in aquaculture industry for pH regulation of pond soil before initiating culture. There is interest in understanding whether or not it can affect pesticide adsorption and desorption in calcareous soil.
Household cleaning
Calcium carbonate is a key ingredient in many household cleaning powders likeComet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
and is used as a scrubbing agent.
Pollution mitigation
In 1989, a researcher, Ken Simmons, introduced into the Whetstone Brook inMassachusetts
Massachusetts ( ; ), officially the Commonwealth of Massachusetts, is a U.S. state, state in the New England region of the Northeastern United States. It borders the Atlantic Ocean and the Gulf of Maine to its east, Connecticut and Rhode ...
. His hope was that the calcium carbonate would counter the acid in the stream from acid rain and save the trout that had ceased to spawn. Although his experiment was a success, it did increase the amount of aluminium ions in the area of the brook that was not treated with the limestone. This shows that can be added to neutralize the effects of acid rain in river
A river is a natural stream of fresh water that flows on land or inside Subterranean river, caves towards another body of water at a lower elevation, such as an ocean, lake, or another river. A river may run dry before reaching the end of ...
ecosystems. Currently calcium carbonate is used to neutralize acidic conditions in both soil and water. Since the 1970s, such ''liming'' has been practiced on a large scale in Sweden to mitigate acidification and several thousand lakes and streams are limed repeatedly.
Calcium carbonate is also used in flue-gas desulfurization applications eliminating harmful and emissions from coal and other fossil fuels burnt in large fossil fuel power stations.
Plastics
Calcium carbonate is commonly used in the plastic industry as a filler. When it is incorporated in a plastic material, it can improve the hardness, stiffness, dimensional stability and processability of the material.Calcination equilibrium
Calcination
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally f ...
of limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
using charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ca ...
fires to produce quicklime
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containin ...
has been practiced since antiquity by cultures all over the world. The temperature at which limestone yields calcium oxide is usually given as 825 °C, but stating an absolute threshold is misleading. Calcium carbonate exists in equilibrium with calcium oxide and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
at any temperature. At each temperature there is a partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of carbon dioxide that is in equilibrium with calcium carbonate. At room temperature the equilibrium overwhelmingly favors calcium carbonate, because the equilibrium pressure is only a tiny fraction of the partial pressure in air, which is about 0.035 kPa.
At temperatures above 550 °C the equilibrium pressure begins to exceed the pressure in air. So above 550 °C, calcium carbonate begins to outgas into air. However, in a charcoal fired kiln, the concentration of will be much higher than it is in air. Indeed, if all the oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in the kiln is consumed in the fire, then the partial pressure of in the kiln can be as high as 20 kPa.
The table shows that this partial pressure is not achieved until the temperature is nearly 800 °C. For the outgassing of from calcium carbonate to happen at an economically useful rate, the equilibrium pressure must significantly exceed the ambient pressure of . And for it to happen rapidly, the equilibrium pressure must exceed total atmospheric pressure of 101 kPa, which happens at 898 °C.
:
Solubility
With varying pressure
 Calcium carbonate is poorly soluble in pure water (47 mg/L at normal atmospheric partial pressure as shown below).
The equilibrium of its solution is given by the equation (with dissolved calcium carbonate on the right):
:
where the solubility product for is given as anywhere from ''K''sp = to ''K''sp = at 25 °C, depending upon the data source. What the equation means is that the product of molar concentration of calcium ions ( moles of dissolved per liter of solution) with the molar concentration of dissolved cannot exceed the value of ''K''sp. This seemingly simple solubility equation, however, must be taken along with the more complicated equilibrium of
Calcium carbonate is poorly soluble in pure water (47 mg/L at normal atmospheric partial pressure as shown below).
The equilibrium of its solution is given by the equation (with dissolved calcium carbonate on the right):
:
where the solubility product for is given as anywhere from ''K''sp = to ''K''sp = at 25 °C, depending upon the data source. What the equation means is that the product of molar concentration of calcium ions ( moles of dissolved per liter of solution) with the molar concentration of dissolved cannot exceed the value of ''K''sp. This seemingly simple solubility equation, however, must be taken along with the more complicated equilibrium of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
with water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
(see carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
). Some of the combines with in the solution according to
:
is known as the bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
ion. Calcium bicarbonate is many times more soluble in water than calcium carbonate—indeed it exists ''only'' in solution.
Some of the combines with in solution according to
:
Some of the breaks up into water and dissolved carbon dioxide according to
:
And dissolved carbon dioxide is in equilibrium with atmospheric carbon dioxide according to
:
For ambient air, ''P'' is around atm (or equivalently 35 Pa). The last equation above fixes the concentration of dissolved as a function of ''P'', independent of the concentration of dissolved . At atmospheric partial pressure of , dissolved concentration is moles per liter. The equation before that fixes the concentration of as a function of concentration. For [] = , it results in = moles per liter. When is known, the remaining three equations together with
:
(which is true for all aqueous solutions), and the constraint that the solution must be electrically neutral, i.e., the overall charge of dissolved positive ions must be cancelled out by the overall charge of dissolved negative ions , make it possible to solve simultaneously for the remaining five unknown concentrations (the previously mentioned form of the neutrality is valid only if calcium carbonate has been put in contact with pure water or with a neutral pH solution; in the case where the initial water solvent pH is not neutral, the balance is not neutral).
The adjacent table shows the result for and (in the form of pH) as a function of ambient partial pressure of (''K''sp = has been taken for the calculation).
* At atmospheric levels of ambient the table indicates that the solution will be slightly alkaline with a maximum solubility of 47 mg/L.
* As ambient partial pressure is reduced below atmospheric levels, the solution becomes more and more alkaline. At extremely low ''P'', dissolved , bicarbonate ion, and carbonate ion largely evaporate from the solution, leaving a highly alkaline solution of calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
, which is more soluble than . For ''P'' = 10−12 atm, the product is still below the solubility product of (). For still lower pressure, precipitation will occur before precipitation.
* As ambient partial pressure increases to levels above atmospheric, pH drops, and much of the carbonate ion is converted to bicarbonate ion, which results in higher solubility of .
The effect of the latter is especially evident in day-to-day life of people who have hard water. Water in aquifers underground can be exposed to levels of much higher than atmospheric. As such, water percolates through calcium carbonate rock, the dissolves according to one of the trends above. When that same water then emerges from the tap, in time, it comes into equilibrium with levels in the air by outgassing its excess . The calcium carbonate becomes less soluble as a result, and the excess precipitates as lime scale. This same process is responsible for the formation of stalactites
A stalactite (, ; , ) is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension, or is ca ...
and stalagmite
A stalagmite (, ; ; )
is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist ...
s in limestone caves.
Two hydrated phases of calcium carbonate, monohydrocalcite
Monohydrocalcite is a mineral that is a hydrous form of calcium carbonate, CaCO3·H2O. It was formerly also known by the name hydrocalcite, which is now discredited by the IMA. It is a trigonal mineral which is white when pure. Monohydrocalcite ...
and ikaite , may precipitate
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemic ...
from water at ambient conditions and persist as metastable phases.
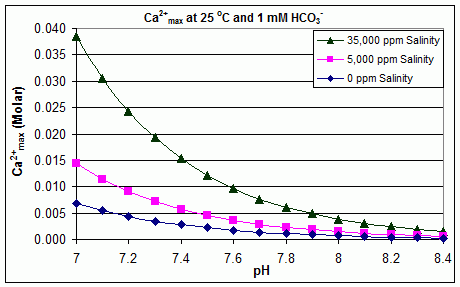
With varying pH, temperature and salinity: scaling in swimming pools
 In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of
In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of sodium bicarbonate
Sodium bicarbonate ( IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply “bicarb” especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cat ...
() to the concentration of about 2 mmol/L as a buffer, then control of pH through use of HCl, , , NaOH or chlorine formulations that are acidic or basic. In this situation, dissolved inorganic carbon ( total inorganic carbon) is far from equilibrium with atmospheric . Progress towards equilibrium through outgassing of is slowed by
In this situation, the dissociation constants for the much faster reactions
:
allow the prediction of concentrations of each dissolved inorganic carbon species in solution, from the added concentration of (which constitutes more than 90% of Bjerrum plot species from pH 7 to pH 8 at 25 °C in fresh water). Addition of will increase concentration at any pH. Rearranging the equations given above, we can see that = , and [] = . Therefore, when concentration is known, the maximum concentration of ions before scaling through precipitation can be predicted from the formula:
:[]max = ×
The solubility product for (''K''sp) and the dissociation constants for the dissolved inorganic carbon species (including ''K''a2) are all substantially affected by temperature and salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensio ...
, with the overall effect that []max increases from freshwater to saltwater, and decreases with rising temperature, pH, or added bicarbonate level, as illustrated in the accompanying graphs.
The trends are illustrative for pool management, but whether scaling occurs also depends on other factors including interactions with , and other ions in the pool, as well as supersaturation effects. Scaling is commonly observed in electrolytic chlorine generators, where there is a high pH near the cathode surface and scale deposition further increases temperature. This is one reason that some pool operators prefer borate over bicarbonate as the primary pH buffer, and avoid the use of pool chemicals containing calcium.
Solubility in a strong or weak acid solution
Solutions ofstrong
Strong may refer to:
Education
* The Strong, an educational institution in Rochester, New York, United States
* Strong Hall (Lawrence, Kansas), an administrative hall of the University of Kansas
* Strong School, New Haven, Connecticut, United ...
( HCl), moderately strong ( sulfamic) or weak
Weak may refer to:
Songs
* Weak (AJR song), "Weak" (AJR song), 2016
* Weak (Melanie C song), "Weak" (Melanie C song), 2011
* Weak (SWV song), "Weak" (SWV song), 1993
* Weak (Skunk Anansie song), "Weak" (Skunk Anansie song), 1995
* "Weak", a son ...
( acetic, citric, sorbic, lactic, phosphoric) acids are commercially available. They are commonly used as descaling agent
A descaling agent or chemical descaler is a liquid chemical substance used to remove limescale from metal surfaces in contact with hot water, such as in boilers, water heaters, and kettles. Limescale is either white or brown in colour due to ...
s to remove limescale
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially that for hot water. It is also often found as a similar deposit on the inner surfaces of old ...
deposits. The maximum amount of that can be "dissolved" by one liter of an acid solution can be calculated using the above equilibrium equations.
* In the case of a strong monoacid with decreasing acid concentration = [], we obtain (with molar mass = 100 g/mol):
::
:where the initial state is the acid solution with no (not taking into account possible dissolution) and the final state is the solution with saturated . For strong acid concentrations, all species have a negligible concentration in the final state with respect to and so that the neutrality equation reduces approximately to 2[] = [] yielding [] ≈ 0.5 []. When the concentration decreases, [] becomes non-negligible so that the preceding expression is no longer valid. For vanishing acid concentrations, one can recover the final pH and the solubility of in pure water.
* In the case of a weak monoacid (here we take acetic acid with p''K''a = 4.76) with decreasing total acid concentration = [] + [AH], we obtain:
::
:For the same total acid concentration, the initial pH of the weak acid is less acid than that of the strong acid; however, the maximum amount of which can be dissolved is approximately the same. This is because in the final state, the pH is larger than the p''K''a, so that the weak acid is almost completely dissociated, yielding in the end as many ions as the strong acid to "dissolve" the calcium carbonate.
* The calculation in the case of phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
(which is the most widely used for domestic applications) is more complicated since the concentrations of the four dissociation states corresponding to this acid must be calculated together with [], [], [], [] and []. The system may be reduced to a seventh degree equation for [] the numerical solution of which gives
::
:where = is the total acid concentration. Thus phosphoric acid is more efficient than a monoacid since at the final almost neutral pH, the second dissociated state concentration [] is not negligible (see phosphoric acid#pH and composition of a phosphoric acid aqueous solution, phosphoric acid).
See also
* Cuttlebone * Cuttlefish * Gesso *Limescale
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially that for hot water. It is also often found as a similar deposit on the inner surfaces of old ...
* Marble
Marble is a metamorphic rock consisting of carbonate minerals (most commonly calcite (CaCO3) or Dolomite (mineral), dolomite (CaMg(CO3)2) that have recrystallized under the influence of heat and pressure. It has a crystalline texture, and is ty ...
* Ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
* Whiting event
* List of climate engineering topics
* Lysocline
References
External links
* * *ATC codes
The Anatomical Therapeutic Chemical (ATC) Classification System is a drug classification system that classifies the active ingredients of drugs according to the organ or system on which they act and their therapeutic, pharmacological and chemica ...
: and
The British Calcium Carbonate Association – What is calcium carbonate
{{Authority control Calcium compounds Carbonates Limestone Phosphate binders Excipients Antacids Food stabilizers E-number additives