|
Limescale
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially that for hot water. It is also often found as a similar deposit on the inner surfaces of old pipes and other surfaces where hard water has flowed. Limescale also forms as travertine or tufa in hard water springs. The colour varies from off-white through a range of greys and pink or reddish browns, depending on the other minerals present. Iron compounds give the reddish-browns. In addition to being unsightly and hard to clean, limescale can seriously damage or impair the operation of various plumbing and heating components.Hermann Weingärtner, "Water" in ''Ullmann's Encyclopedia of Industrial Chemistry'', December 2006, Wiley–VCH, Weinheim. Descaling agents are commonly used to remove limescale. Prevention of fouling by scale build-up relies on the technologies of water softening or other water treatment. Limescale can als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Descaling Agent
A descaling agent or chemical descaler is a liquid chemical substance used to remove limescale from metal surfaces in contact with hot water, such as in boilers, water heaters, and kettles. Limescale is either white or brown in colour due to the presence of iron compounds. Glass surfaces may also exhibit scaling stains, as can many ceramic surfaces present in bathrooms and kitchen, and descaling agents can be used safely to remove those stains without affecting the substrate since both ceramics and glass are unreactive to most acids. Action Descaling agents are typically acidic compounds such as hydrochloric acid that react with the calcium carbonate and magnesium carbonate compounds present in the scale, producing carbon dioxide gas and a soluble salt. : : Strongly acidic descaling agents are usually corrosive to the eyes and skin and can also attack and degrade clothing fibres, so appropriate protection such as rubber gloves and plastic aprons should be used in cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hard Water
Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates. Drinking hard water may have moderate health benefits. It can pose critical problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. In domestic settings, hard water is often indicated by a lack of foam formation when soap is agitated in water, and by the formation of limescale in kettles and water heaters. Wherever water hardness is a concern, water softening is commonly used to reduce hard water's adverse effects. Origins Natural rainwater, snow and other forms of precipitation typically have low concentrations of divalent cations such as calcium and magnesium. They may have small concentrations of ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
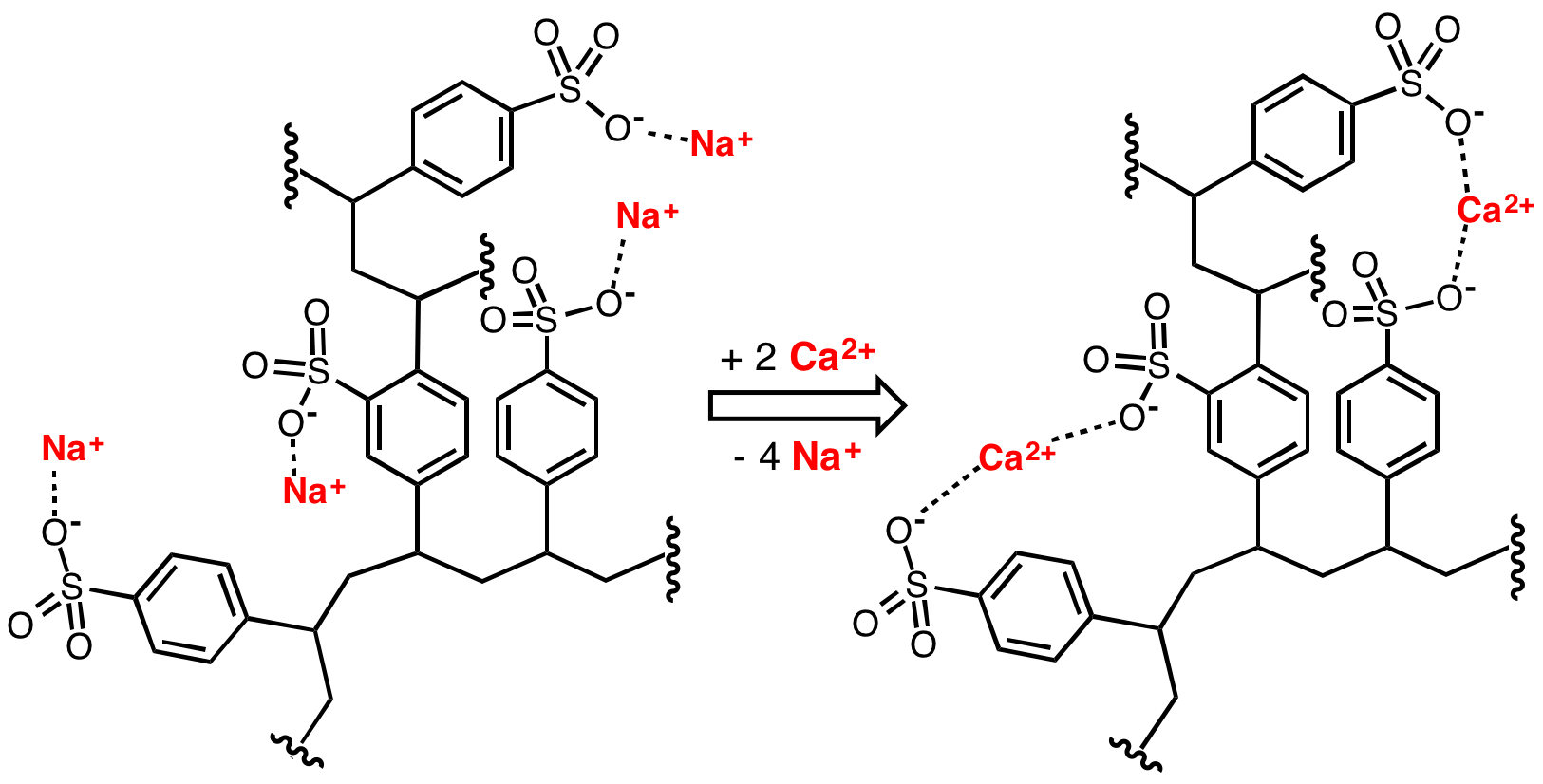
Water Softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating Fouling#Precipitation fouling, scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins, but is increasingly being accomplished using nanofiltration or reverse osmosis membranes. Rationale The presence of certain metal ions like calcium and magnesium, principally as bicarbonates, chlorides, and sulfates, in water causes a variety of problems. Hard water leads to the buildup of limescale, which can foul plumbing, and promote galvanic corrosion. In industrial scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems. The slippery feeling associ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Heating
Water heating is a heat transfer process that uses an energy source to heat water above its initial temperature. Typical domestic uses of hot water include cooking, cleaning, bathing, and space heating. In industry, hot water and water heated to steam have many uses. Domestically, water is traditionally heated in vessels known as ''water heaters'', ''kettles'', ''cauldrons'', ''pots'', or ''coppers''. These metal vessels that heat a batch of water do not produce a continual supply of heated water at a preset temperature. Rarely, hot water occurs naturally, usually from natural hot springs. The temperature varies with the consumption rate, becoming cooler as flow increases. Appliances that provide a continual supply of hot water are called ''water heaters'', ''hot water heaters'', ''hot water tanks'', ''boilers'', ''heat exchangers'', ''geysers'' (Southern Africa and the Arab world), or ''calorifiers''. These names depend on region, and whether they heat Drinking water, potable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiler
A boiler is a closed vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central heating, boiler-based power generation, cooking, and sanitation. Heat sources In a fossil fuel power plant using a steam cycle for power generation, the primary heat source will be combustion of coal, oil, or natural gas. In some cases byproduct fuel such as the carbon monoxide rich offgasses of a coke battery can be burned to heat a boiler; biofuels such as bagasse, where economically available, can also be used. In a nuclear power plant, boilers called steam generators are heated by the heat produced by nuclear fission. Where a large volume of hot gas is available from some process, a heat recovery steam generator or recovery boiler can use the heat to produce steam, with little or no extra fuel consumed; such a configuration is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling, organic) or a non-living substance (inorganic). Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function. Other terms used in the literature to describe fouling include deposit formation, encrustation, crudding, deposition, scaling, scale formation, slagging, and sludge formation. The last six terms have a more narrow meaning than fouling within the scope of the fouling science and technology, and they also have meanings outside of this scope; therefore, they should be used with caution. Fouling phenomena are common and diverse, ranging from fouling of ship hulls, natural surfaces in the marine environment (fouling community, marine fouling), fouling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water. All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates. These hard waters tend to form carbonate scale in pipes and boilers, and they react with soaps to form an undesirable scum. Attempts to prepare compounds such as solid calcium bicarbonate by evaporating its solution t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a wikt:saturated#Chemistry, saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscibility, miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the Thermodynamic system, system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of ''equilibrium chemistry''. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |