Water is an
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the
chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. It is a transparent, tasteless, odorless, and
nearly colorless chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
. It is the main constituent of Earth's
hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
and the
fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
s of all known living organisms (in which it acts as a
solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
). It is vital for all known forms of life, despite not providing
food energy
Food energy is chemical energy that animals and humans derive from food to sustain their metabolism and muscular activity.
Most animals derive most of their energy from aerobic respiration, namely combining the carbohydrates, fats, and protein ...
or organic
micronutrient
Micronutrients are essential chemicals required by organisms in small quantities to perform various biogeochemical processes and regulate physiological functions of cells and organs. By enabling these processes, micronutrients support the heal ...
s. Its chemical formula, , indicates that each of its
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s contains one
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and two
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s, connected by
covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at
standard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
.
Because Earth's environment is relatively close to water's
triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
, water exists on Earth as a solid, a liquid, and a gas. It forms
precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
in the form of rain and
aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be generated from natural or Human impact on the environment, human causes. The term ''aerosol'' co ...
s in the form of
fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided,
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line ice may precipitate in the form of snow. The gaseous state of water is
steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is inv ...
or
water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
.
Water covers about 71.0% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%).
Small portions of water occur as
groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
(1.7%), in the
glaciers
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires ...
and the
ice caps
In glaciology, an ice cap is a mass of ice that covers less than of land
Land, also known as dry land, ground, or earth, is the solid terrestrial surface of Earth not submerged by the ocean or another body of water. It makes up 29.2% ...
of
Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean (also known as the Antarctic Ocean), it contains the geographic South Pole. ...
and Greenland (1.7%), and in the air as
vapor
In physics, a vapor (American English) or vapour (Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R ...
, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%).
Water moves continually through the
water cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fai ...
of
evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
,
transpiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. It is a passive process that requires no energy expense by the plant. Transpiration also cools plants, c ...
(
evapotranspiration
Evapotranspiration (ET) refers to the combined processes which move water from the Earth's surface (open water and ice surfaces, bare soil and vegetation) into the Atmosphere of Earth, atmosphere. It covers both water evaporation (movement of w ...
),
condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
,
precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
, and
runoff, usually reaching the sea.
Water plays an important role in the
world economy
The world economy or global economy is the economy of all humans in the world, referring to the global economic system, which includes all economic activities conducted both within and between nations, including production (economics), producti ...
. Approximately 70% of the
fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salt (chemistry), salts and other total dissolved solids. The term excludes seawater and brackish water, but it does include ...
used by humans
goes to agriculture.
Fishing in
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
and
fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salt (chemistry), salts and other total dissolved solids. The term excludes seawater and brackish water, but it does include ...
bodies has been, and continues to be, a major source of food for many parts of the world, providing 6.5% of global protein. Much of the long-distance trade of commodities (such as oil, natural gas, and manufactured products) is
transported by boats through seas, rivers, lakes, and canals. Large quantities of water, ice, and steam are used for cooling and heating in industry and homes. Water is an excellent solvent for a wide variety of substances, both mineral and organic; as such, it is widely used in industrial processes and in cooking and washing. Water, ice, and snow are also central to many sports and other forms of entertainment, such as swimming, pleasure boating,
boat racing
Boat racing is a sport in which boats, or other types of watercraft, race on water body, water. Boat racing powered by oars is recorded as having occurred in ancient Egypt, and it is likely that people have engaged in races involving boats and ...
,
surfing
Surfing is a surface water sport in which an individual, a surfer (or two in tandem surfing), uses a board to ride on the forward section, or face, of a moving wave of water, which usually carries the surfer towards the shore. Waves suita ...
,
sport fishing,
diving
Diving most often refers to:
* Diving (sport), the sport of jumping into deep water
* Underwater diving, human activity underwater for recreational or occupational purposes
Diving or Dive may also refer to:
Sports
* Dive (American football), ...
,
ice skating
Ice skating is the Human-powered transport, self-propulsion and gliding of a person across an ice surface, using metal-bladed ice skates. People skate for various reasons, including recreation (fun), exercise, competitive sports, and commuting. ...
,
snowboarding
Snowboarding is a recreational and competitive activity that involves descending a snow-covered surface while standing on a snowboard that is almost always attached to a rider's feet. It features in the Winter Olympic Games and Winter Paralym ...
, and
skiing
Skiing is the use of skis to glide on snow for basic transport, a recreational activity, or a competitive winter sport. Many types of competitive skiing events are recognized by the International Olympic Committee (IOC), and the International S ...
.
Etymology
The word ''water'' comes from
Old English
Old English ( or , or ), or Anglo-Saxon, is the earliest recorded form of the English language, spoken in England and southern and eastern Scotland in the Early Middle Ages. It developed from the languages brought to Great Britain by Anglo-S ...
, from
Proto-Germanic
Proto-Germanic (abbreviated PGmc; also called Common Germanic) is the linguistic reconstruction, reconstructed proto-language of the Germanic languages, Germanic branch of the Indo-European languages.
Proto-Germanic eventually developed from ...
(source also of
Old Saxon
Old Saxon (), also known as Old Low German (), was a Germanic language and the earliest recorded form of Low German (spoken nowadays in Northern Germany, the northeastern Netherlands, southern Denmark, the Americas and parts of Eastern Eur ...
,
Old Frisian
Old Frisian was a West Germanic language spoken between the late 13th century and the end of 16th century. It is the common ancestor of all the modern Frisian languages except for the North Frisian language#Insular North Frisian, Insular North ...
,
Dutch ,
Old High German
Old High German (OHG; ) is the earliest stage of the German language, conventionally identified as the period from around 500/750 to 1050. Rather than representing a single supra-regional form of German, Old High German encompasses the numerous ...
, German , ,
Gothic ()), from
Proto-Indo-European
Proto-Indo-European (PIE) is the reconstructed common ancestor of the Indo-European language family. No direct record of Proto-Indo-European exists; its proposed features have been derived by linguistic reconstruction from documented Indo-Euro ...
, suffixed form of root (; ). Also
cognate
In historical linguistics, cognates or lexical cognates are sets of words that have been inherited in direct descent from an etymological ancestor in a common parent language.
Because language change can have radical effects on both the s ...
, through the Indo-European root, with Greek (; from Ancient Greek (), whence English ), Russian (), Irish , and
Albanian .
History
On Earth
Properties
Water () is a
polar inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
. At
room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
it is a
taste
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste. Taste is the perception stimulated when a substance in the mouth biochemistry, reacts chemically with taste receptor cells l ...
less and
odor
An odor (American English) or odour ( Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive ...
less liquid, nearly
colorless with a
hint of blue. The simplest
hydrogen chalcogenide, it is by far the most studied chemical compound and is sometimes described as the "universal solvent" for its ability to dissolve more substances than any other liquid, though it is poor at dissolving nonpolar substances. This allows it to be the "
solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
of life": indeed, water as found in nature almost always includes various dissolved substances, and special steps are required to obtain chemically
pure water
Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently pur ...
. Water is the only common substance to exist as a solid, liquid, and gas in normal terrestrial conditions.
States
Along with ''oxidane'', ''water'' is one of the two official names for the chemical compound ; it is also the liquid phase of .
The other two common
states of matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma.
Different states are distinguished by the ways the component parti ...
of water are the solid phase, ice, and the gaseous phase,
water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
or
steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is inv ...
. The addition or removal of heat can cause
phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s:
freezing
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point.
For most substances, the melting and freezing points are the same temperature; however, certain substances possess dif ...
(water to ice),
melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
(ice to water),
vaporization
Vaporization (or vapo(u)risation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenome ...
(water to vapor),
condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
(vapor to water),
sublimation (ice to vapor) and
deposition (vapor to ice).
Density
Water is one of only a few common naturally occurring substances which, for some temperature ranges, become less
dense
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be use ...
as they cool, and the only known naturally occurring substance which does so while liquid. In addition it is unusual as it becomes significantly less
dense
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be use ...
as it freezes, though it is not unique in that respect.
At 1 atm pressure, it reaches its maximum density of at .
Below that temperature, but above the freezing point of , it expands becoming less dense until it reaches freezing point, reaching a density in its liquid phase of .
Once it freezes and becomes ice, it expands by about 9%, with a density of . This expansion can exert enormous pressure, bursting pipes and cracking rocks.
As a solid, it displays the usual behavior of contracting and becoming more dense as it cools. These unusual thermal properties have important consequences for life on earth.
In a lake or ocean, water at sinks to the bottom, and ice forms on the surface, floating on the liquid water. This ice insulates the water below, preventing it from freezing solid. Without this protection, most aquatic organisms residing in lakes would perish during the winter. In addition, this anomalous behavior is an important part of the
thermohaline circulation
Thermohaline circulation (THC) is a part of the large-scale Ocean current, ocean circulation driven by global density gradients formed by surface heat and freshwater fluxes. The name ''thermohaline'' is derived from ''wikt:thermo-, thermo-'', r ...
which distributes heat around the planet's oceans.
Magnetism
Water is a
diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
material.
Though interaction is weak, with superconducting magnets it can attain a notable interaction.
Phase transitions
At a pressure of one
atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
(atm), ice melts or water freezes (solidifies) at and water boils or vapor condenses at . However, even below the boiling point, water can change to vapor at its surface by
evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
(vaporization throughout the liquid is known as
boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor, vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to ...
). Sublimation and deposition also occur on surfaces.
[ For example, ]frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor that deposits onto a freezing surface. Frost forms when the air contains more water vapor than it can normally hold at a specific temperature. The process is simila ...
is deposited on cold surfaces while snowflake
A snowflake is a single ice crystal that is large enough to fall through the Earth's atmosphere as snow.Knight, C.; Knight, N. (1973). Snow crystals. Scientific American, vol. 228, no. 1, pp. 100–107.Hobbs, P.V. 1974. Ice Physics. Oxford: C ...
s form by deposition on an aerosol particle or ice nucleus. In the process of freeze-drying
Freeze drying, also known as lyophilization or cryodesiccation, is a low temperature Food drying, dehydration process that involves freezing the product and lowering pressure, thereby removing the ice by Sublimation (phase transition), sublimat ...
, a food is frozen and then stored at low pressure so the ice on its surface sublimates.Clausius–Clapeyron relation
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is nam ...
:
where and are the molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume (''V'') occupied by a substance to the amount of substance (''n''), usually at a given temperature and pressure. It is also eq ...
s of the liquid and solid phases, and is the molar latent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation. ...
of melting. In most substances, the volume increases when melting occurs, so the melting temperature increases with pressure. However, because ice is less dense than water, the melting temperature decreases.subglacial lake
A subglacial lake is a lake that is found under a glacier, typically beneath an ice cap or ice sheet. Subglacial lakes form at the boundary between ice and the underlying bedrock, where liquid water can exist above the lower melting point of ic ...
s.
The Clausius-Clapeyron relation also applies to the boiling point, but with the liquid/gas transition the vapor phase has a much lower density than the liquid phase, so the boiling point increases with pressure. Water can remain in a liquid state at high temperatures in the deep ocean or underground. For example, temperatures exceed in Old Faithful
Old Faithful is a cone geyser in Yellowstone National Park in Wyoming, United States. It was named in 1870 during the Washburn–Langford–Doane Expedition and was the first geyser in the park to be named. It is a highly predictable geotherma ...
, a geyser in Yellowstone National Park
Yellowstone National Park is a List of national parks of the United States, national park of the United States located in the northwest corner of Wyoming, with small portions extending into Montana and Idaho. It was established by the 42nd U ...
. In hydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s, the temperature can exceed .
At sea level, the boiling point of water is . As atmospheric pressure decreases with altitude, the boiling point decreases by 1 °C every 274 meters. High-altitude cooking
High-altitude cooking is cooking done at altitudes that are considerably higher than sea level. At elevated altitudes, any cooking that involves boiling or steaming generally requires compensation for lower temperatures because the boiling point ...
takes longer than sea-level cooking. For example, at , cooking time must be increased by a fourth to achieve the desired result. Conversely, a pressure cooker
A pressure cooker is a sealed vessel for cooking food with the use of high pressure steam and water or a water-based liquid, a process called pressure cooking. The high pressure limits boiling and creates higher temperatures not possible at low ...
can be used to decrease cooking times by raising the boiling temperature. In a vacuum, water will boil at room temperature.
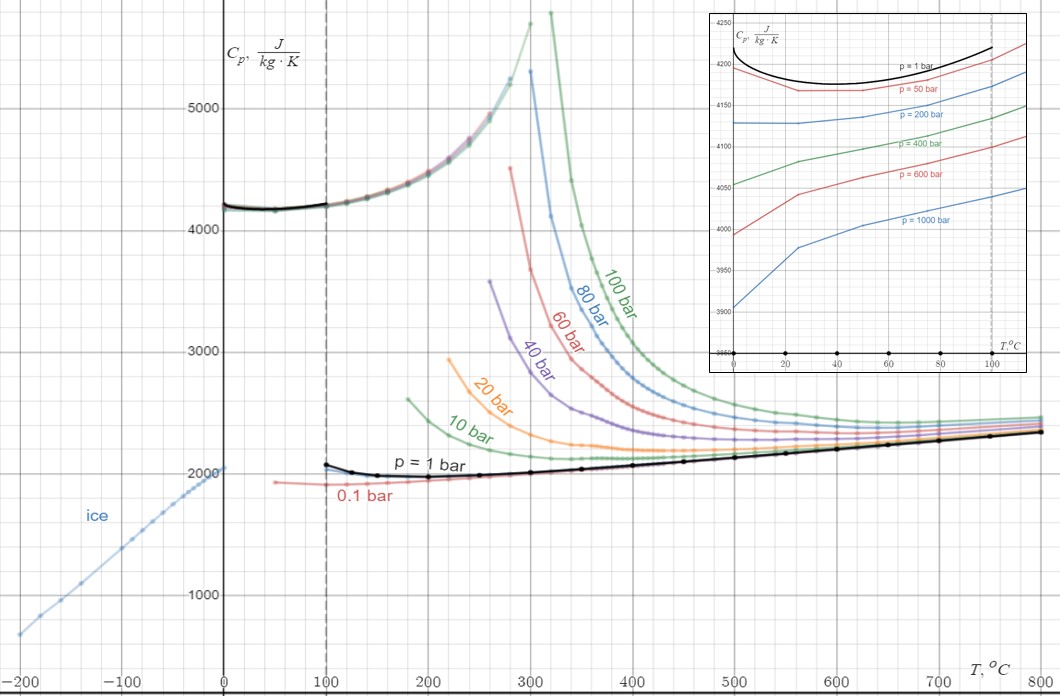
Triple and critical points
 On a pressure/temperature phase diagram (see figure), there are curves separating solid from vapor, vapor from liquid, and liquid from solid. These meet at a single point called the
On a pressure/temperature phase diagram (see figure), there are curves separating solid from vapor, vapor from liquid, and liquid from solid. These meet at a single point called the triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
, where all three phases can coexist. The triple point is at a temperature of and a pressure of ; it is the lowest pressure at which liquid water can exist. Until 2019, the triple point was used to define the Kelvin temperature scale
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
.supercritical fluid
A supercritical fluid (SCF) is a substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous sol ...
. It can be gradually compressed or expanded between gas-like and liquid-like densities; its properties (which are quite different from those of ambient water) are sensitive to density. For example, for suitable pressures and temperatures it can mix freely with nonpolar compounds, including most organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s. This makes it useful in a variety of applications including high-temperature electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
and as an ecologically benign solvent or catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
in chemical reactions involving organic compounds. In Earth's mantle, it acts as a solvent during mineral formation, dissolution and deposition.
Phases of ice and water
The normal form of ice on the surface of Earth is ice Ih, a phase that forms crystals with hexagonal symmetry. Another with cubic crystalline symmetry, ice Ic, can occur in the upper atmosphere. As the pressure increases, ice forms other crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
s. As of 2024, twenty have been experimentally confirmed and several more are predicted theoretically. The eighteenth form of ice, ice XVIII, a face-centred-cubic, superionic ice phase, was discovered when a droplet of water was subject to a shock wave that raised the water's pressure to millions of atmospheres and its temperature to thousands of degrees, resulting in a structure of rigid oxygen atoms in which hydrogen atoms flowed freely.graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
, ice forms a square lattice.
The details of the chemical nature of liquid water are not well understood; some theories suggest that its unusual behavior is due to the existence of two liquid states.
Taste and odor
Pure water is usually described as tasteless and odorless, although humans have specific sensors that can feel the presence of water in their mouths,[Edmund T. Rolls (2005). ''Emotion Explained''. Oxford University Press, Medical. .] and frogs are known to be able to smell it.[R. Llinas, W. Precht (2012), ''Frog Neurobiology: A Handbook''. Springer Science & Business Media. ] However, water from ordinary sources (including mineral water
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. It is usually still, but may be sparkling ( carbonated/ effervescent).
Traditionally, mineral waters were used or consumed at t ...
) usually has many dissolved substances that may give it varying tastes and odors. Humans and other animals have developed senses that enable them to evaluate the potability
Drinking water or potable water is water that is safe for ingestion, either when drinking, drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is al ...
of water to avoid water that is too salty or putrid.
Color and appearance
Pure water is visibly blue due to absorption of light in the region c. 600–800 nm. The color can be easily observed in a glass of tap-water placed against a pure white background, in daylight. The principal absorption bands responsible for the color are overtone
An overtone is any resonant frequency above the fundamental frequency of a sound. (An overtone may or may not be a harmonic) In other words, overtones are all pitches higher than the lowest pitch within an individual sound; the fundamental i ...
s of the O–H stretching vibrations. The apparent intensity of the color increases with the depth of the water column, following Beer's law. This also applies, for example, with a swimming pool when the light source is sunlight reflected from the pool's white tiles.
In nature, the color may also be modified from blue to green due to the presence of suspended solids or algae.
In industry, near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research inc ...
is used with aqueous solutions as the greater intensity of the lower overtones of water means that glass cuvette
In laboratories, a cuvette () is a small tube-like container with straight sides and a circular or square cross-section. It is sealed at one end, and made of a clear, transparent material such as plastic, glass, or fused quartz. Cuvettes are des ...
s with short path-length may be employed. To observe the fundamental stretching absorption spectrum of water or of an aqueous solution in the region around 3,500 cm (2.85 μm) a path length of about 25 μm is needed. Also, the cuvette must be both transparent around 3500 cm and insoluble in water; calcium fluoride is one material that is in common use for the cuvette windows with aqueous solutions.
The Raman-active fundamental vibrations may be observed with, for example, a 1 cm sample cell.
Aquatic plant
Aquatic plants, also referred to as hydrophytes, are vascular plants and Non-vascular plant, non-vascular plants that have adapted to live in aquatic ecosystem, aquatic environments (marine ecosystem, saltwater or freshwater ecosystem, freshwater ...
s, algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
, and other photosynthetic
Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ...
organisms can live in water up to hundreds of meters deep, because sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
can reach them.
Practically no sunlight reaches the parts of the oceans below of depth.
The refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
of liquid water (1.333 at ) is much higher than that of air (1.0), similar to those of alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
s and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, but lower than those of glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
(1.473), benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
(1.501), carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
(1.627), and common types of glass (1.4 to 1.6). The refraction index of ice (1.31) is lower than that of liquid water.
Molecular polarity
 In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are '' lone pairs'' of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms. The O–H bond length is about 0.096 nm.
Other substances have a tetrahedral molecular structure, for example
In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are '' lone pairs'' of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms. The O–H bond length is about 0.096 nm.
Other substances have a tetrahedral molecular structure, for example methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
() and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(). However, oxygen is more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than most other elements, so the oxygen atom has a negative partial charge while the hydrogen atoms are partially positively charged. Along with the bent structure, this gives the molecule an electrical dipole moment and it is classified as a polar molecule
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
.
Water is a good polar solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
, dissolving many salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
and hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
organic molecules such as sugars and simple alcohols such as ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
. Water also dissolves many gases, such as oxygen and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
—the latter giving the fizz of carbonated beverages, sparkling wine
Sparkling wine is a wine with significant levels of carbon dioxide in it, making it fizzy. While it is common to refer to this as champagne, European Union countries legally reserve that word for products exclusively produced in the Champagne ( ...
s and beers. In addition, many substances in living organisms, such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s, are dissolved in water. The interactions between water and the subunits of these biomacromolecules shape protein folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, t ...
, DNA base pairing, and other phenomena crucial to life (hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and to be excluded by water. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpola ...
).
Many organic substances (such as fats and oils and alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
) are hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
, that is, insoluble in water. Many inorganic substances are insoluble too, including most metal oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s, sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s, and silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
s.
Hydrogen bonding
 Because of its polarity, a molecule of water in the liquid or solid state can form up to four
Because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a mo ...
with neighboring molecules. Hydrogen bonds are about ten times as strong as the Van der Waals force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical elec ...
that attracts molecules to each other in most liquids. This is the reason why the melting and boiling points of water are much higher than those of other analogous compounds like hydrogen sulfide. They also explain its exceptionally high specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat ...
(about 4.2 J/(g·K)), heat of fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a s ...
(about 333 J/g), heat of vaporization
In thermodynamics, the enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to Phase transition, transform a qua ...
(), and thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
(between 0.561 and 0.679 W/(m·K)). These properties make water more effective at moderating Earth's climate
Climate is the long-term weather pattern in a region, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteoro ...
, by storing heat and transporting it between the oceans and the atmosphere. The hydrogen bonds of water are around 23 kJ/mol (compared to a covalent O–H bond at 492 kJ/mol). Of this, it is estimated that 90% is attributable to electrostatics, while the remaining 10% is partially covalent.
These bonds are the cause of water's high surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
and capillary forces. The capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of external forces like Gravitation, gravity.
The effe ...
refers to the tendency of water to move up a narrow tube against the force of gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force b ...
. This property is relied upon by all vascular plant
Vascular plants (), also called tracheophytes (, ) or collectively tracheophyta (; ), are plants that have lignin, lignified tissues (the xylem) for conducting water and minerals throughout the plant. They also have a specialized non-lignified Ti ...
s, such as trees.
Self-ionization
Water is a weak solution of hydronium hydroxide—there is an equilibrium , in combination with solvation of the resulting hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved ...
and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
ions.
Electrical conductivity and electrolysis
Pure water has a low electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
, which increases with the dissolution of a small amount of ionic material such as common salt.
Liquid water can be split into the elements hydrogen and oxygen by passing an electric current through it—a process called electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
. The decomposition requires more energy input than the heat released by the inverse process (285.8 kJ/ mol, or 15.9 MJ/kg).
Mechanical properties
Liquid water can be assumed to be incompressible for most purposes: its compressibility ranges from 4.4 to in ordinary conditions. Even in oceans at 4 km depth, where the pressure is 400 atm, water suffers only a 1.8% decrease in volume.viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
of water is about 10 Pa· s or 0.01 poise at , and the speed of sound
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elasticity (solid mechanics), elastic medium. More simply, the speed of sound is how fast vibrations travel. At , the speed of sound in a ...
in liquid water ranges between depending on temperature. Sound travels long distances in water with little attenuation
In physics, attenuation (in some contexts, extinction) is the gradual loss of flux intensity through a Transmission medium, medium. For instance, dark glasses attenuate sunlight, lead attenuates X-rays, and water and air attenuate both light and ...
, especially at low frequencies (roughly 0.03 dB/km for 1 k Hz), a property that is exploited by cetaceans
Cetacea (; , ) is an infraorder of aquatic mammals belonging to the order Artiodactyla that includes whales, dolphins and porpoises. Key characteristics are their fully aquatic lifestyle, streamlined body shape, often large size and exclusively c ...
and humans for communication and environment sensing (sonar
Sonar (sound navigation and ranging or sonic navigation and ranging) is a technique that uses sound propagation (usually underwater, as in submarine navigation) to navigate, measure distances ( ranging), communicate with or detect objects o ...
).[UK National Physical Laboratory]
Calculation of absorption of sound in seawater
. Online site, last accessed on 28 September 2016.
Reactivity
Metallic elements which are more electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than hydrogen, particularly the alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and alkaline earth metals
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are a ...
such as lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
and caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
displace hydrogen from water, forming hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
s and releasing hydrogen. At high temperatures, carbon reacts with steam to form carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and hydrogen.
On Earth
Hydrology is the study of the movement, distribution, and quality of water throughout the Earth. The study of the distribution of water is hydrography
Hydrography is the branch of applied sciences which deals with the measurement and description of the physical features of oceans, seas, coastal areas, lakes and rivers, as well as with the prediction of their change over time, for the primary ...
. The study of the distribution and movement of groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
is hydrogeology
Hydrogeology (''hydro-'' meaning water, and ''-geology'' meaning the study of the Earth) is the area of geology that deals with the distribution and movement of groundwater in the soil and rock (geology), rocks of the Earth's crust (ge ...
, of glaciers is glaciology
Glaciology (; ) is the scientific study of glaciers, or, more generally, ice and natural phenomena that involve ice.
Glaciology is an interdisciplinary Earth science that integrates geophysics, geology, physical geography, geomorphology, clim ...
, of inland waters is limnology
Limnology ( ; ) is the study of inland aquatic ecosystems.
It includes aspects of the biological, chemical, physical, and geological characteristics of fresh and saline, natural and man-made bodies of water. This includes the study of lakes, ...
and distribution of oceans is oceanography
Oceanography (), also known as oceanology, sea science, ocean science, and marine science, is the scientific study of the ocean, including its physics, chemistry, biology, and geology.
It is an Earth science, which covers a wide range of to ...
. Ecological processes with hydrology are in the focus of ecohydrology
Ecohydrology (from Ancient Greek, Greek , ''oikos'', "house(hold)"; , ''hydōr'', "water"; and , ''-logy, -logia'') is an interdisciplinary scientific field studying the interactions between water and ecological systems. It is considered a sub disc ...
.
The collective mass of water found on, under, and over the surface of a planet is called the hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
. Earth's approximate water volume (the total water supply of the world) is .bodies of water
A body of water or waterbody is any significant accumulation of water on the surface of Earth or another planet. The term most often refers to oceans, seas, and lakes, but it includes smaller pools of water such as ponds, wetlands, or more ra ...
, such as an ocean, sea, lake, river, stream, canal
Canals or artificial waterways are waterways or engineered channels built for drainage management (e.g. flood control and irrigation) or for conveyancing water transport vehicles (e.g. water taxi). They carry free, calm surface ...
, pond, or puddle
A puddle is a very small accumulation of liquid, usually water, on a surface. It can form either by pooling in a depression on the surface, or by surface tension upon a flat surface. Puddles are often characterized by murky water or mud due to t ...
. The majority of water on Earth is seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
. Water is also present in the atmosphere in solid, liquid, and vapor states. It also exists as groundwater in aquifer
An aquifer is an underground layer of water-bearing material, consisting of permeability (Earth sciences), permeable or fractured rock, or of unconsolidated materials (gravel, sand, or silt). Aquifers vary greatly in their characteristics. The s ...
s.
Water is important in many geological processes. Groundwater is present in most rocks, and the pressure of this groundwater affects patterns of faulting
In geology, a fault is a planar fracture or discontinuity in a volume of rock across which there has been significant displacement as a result of rock-mass movements. Large faults within Earth's crust result from the action of plate tectonic ...
. Water in the mantle is responsible for the melt that produces volcano
A volcano is commonly defined as a vent or fissure in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most oft ...
es at subduction zone
Subduction is a geological process in which the oceanic lithosphere and some continental lithosphere is recycled into the Earth's mantle at the convergent boundaries between tectonic plates. Where one tectonic plate converges with a second p ...
s. On the surface of the Earth, water is important in both chemical and physical weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
processes. Water, and to a lesser but still significant extent, ice, are also responsible for a large amount of sediment transport
Sediment transport is the movement of solid particles (sediment), typically due to a combination of gravity acting on the sediment, and the movement of the fluid in which the sediment is entrained. Sediment transport occurs in natural systems wh ...
that occurs on the surface of the earth. Deposition of transported sediment forms many types of sedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
s, which make up the geologic record
The geologic record in stratigraphy, paleontology and other natural sciences refers to the entirety of the layers of rock strata. That is, deposits laid down by volcanism or by deposition of sediment derived from weathering detritus (clays, sa ...
of Earth history
The natural history of Earth concerns the development of planet Earth from its formation to the present day. Nearly all branches of natural science have contributed to understanding of the main events of Earth's past, characterized by consta ...
.
Water cycle
 The water cycle (known scientifically as the hydrologic cycle) is the continuous exchange of water within the
The water cycle (known scientifically as the hydrologic cycle) is the continuous exchange of water within the hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
, between the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
water, surface water
Surface water is water located on top of land, forming terrestrial (surrounding by land on all sides) waterbodies, and may also be referred to as ''blue water'', opposed to the seawater and waterbodies like the ocean.
The vast majority of surfac ...
, groundwater, and plants.
Water moves perpetually through each of these regions in the ''water cycle'' consisting of the following transfer processes:
* evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
from oceans and other water bodies into the air and transpiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. It is a passive process that requires no energy expense by the plant. Transpiration also cools plants, c ...
from land plants and animals into the air.
* precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
, from water vapor condensing from the air and falling to the earth or ocean.
* runoff from the land usually reaching the sea.
Most water vapors found mostly in the ocean returns to it, but winds carry water vapor over land at the same rate as runoff into the sea, about 47 Tt per year while evaporation and transpiration happening in land masses also contribute another 72 Tt per year. Precipitation, at a rate of 119 Tt per year over land, has several forms: most commonly rain, snow, and hail
Hail is a form of solid Precipitation (meteorology), precipitation. It is distinct from ice pellets (American English "sleet"), though the two are often confused. It consists of balls or irregular lumps of ice, each of which is called a hailsto ...
, with some contribution from fog and dew
Dew is water in the form of droplets that appears on thin, exposed objects in the morning or evening due to condensation.
As the exposed surface cools by thermal radiation, radiating its heat, atmospheric moisture condenses at a rate grea ...
. Dew is small drops of water that are condensed when a high density of water vapor meets a cool surface. Dew usually forms in the morning when the temperature is the lowest, just before sunrise and when the temperature of the earth's surface starts to increase. Condensed water in the air may also refract
In physics, refraction is the redirection of a wave as it passes from one medium to another. The redirection can be caused by the wave's change in speed or by a change in the medium. Refraction of light is the most commonly observed phenome ...
sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
to produce rainbow
A rainbow is an optical phenomenon caused by refraction, internal reflection and dispersion of light in water droplets resulting in a continuous spectrum of light appearing in the sky. The rainbow takes the form of a multicoloured circular ...
s.
Water runoff often collects over watersheds flowing into rivers. Through erosion
Erosion is the action of surface processes (such as Surface runoff, water flow or wind) that removes soil, Rock (geology), rock, or dissolved material from one location on the Earth's crust#Crust, Earth's crust and then sediment transport, tran ...
, runoff shapes the environment creating river valley
A valley is an elongated low area often running between hills or mountains and typically containing a river or stream running from one end to the other. Most valleys are formed by erosion of the land surface by rivers or streams over ...
s and deltas
A river delta is a landform, wikt:archetype#Noun, archetypically triangular, created by the deposition (geology), deposition of the sediments that are carried by the waters of a river, where the river merges with a body of slow-moving water or ...
which provide rich soil and level ground for the establishment of population centers. A flood occurs when an area of land, usually low-lying, is covered with water which occurs when a river overflows its banks or a storm surge happens. On the other hand, drought is an extended period of months or years when a region notes a deficiency in its water supply. This occurs when a region receives consistently below average precipitation either due to its topography or due to its location in terms of latitude
In geography, latitude is a geographic coordinate system, geographic coordinate that specifies the north-south position of a point on the surface of the Earth or another celestial body. Latitude is given as an angle that ranges from −90° at t ...
.
Water resources
Water resources are natural resource
Natural resources are resources that are drawn from nature and used with few modifications. This includes the sources of valued characteristics such as commercial and industrial use, aesthetic value, scientific interest, and cultural value. ...
s of water that are potentially useful for humans, for example as a source of drinking water supply
Water supply is the provision of water by public utilities, commercial organisations, community endeavors or by individuals, usually via a system of pumps and pipes. Public water supply systems are crucial to properly functioning societies. Th ...
or irrigation
Irrigation (also referred to as watering of plants) is the practice of applying controlled amounts of water to land to help grow crops, landscape plants, and lawns. Irrigation has been a key aspect of agriculture for over 5,000 years and has bee ...
water. Water occurs as both "stocks" and "flows". Water can be stored as lakes, water vapor, groundwater or aquifers, and ice and snow. Of the total volume of global freshwater, an estimated 69 percent is stored in glaciers and permanent snow cover; 30 percent is in groundwater; and the remaining 1 percent in lakes, rivers, the atmosphere, and biota. The length of time water remains in storage is highly variable: some aquifers consist of water stored over thousands of years but lake volumes may fluctuate on a seasonal basis, decreasing during dry periods and increasing during wet ones. A substantial fraction of the water supply for some regions consists of water extracted from water stored in stocks, and when withdrawals exceed recharge, stocks decrease. By some estimates, as much as 30 percent of total water used for irrigation comes from unsustainable withdrawals of groundwater, causing groundwater depletion.
Seawater and tides
Seawater contains about 3.5% sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
on average, plus smaller amounts of other substances. The physical properties of seawater differ from fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salt (chemistry), salts and other total dissolved solids. The term excludes seawater and brackish water, but it does include ...
in some important respects. It freezes at a lower temperature (about ) and its density increases with decreasing temperature to the freezing point, instead of reaching maximum density at a temperature above freezing. The salinity of water in major seas varies from about 0.7% in the Baltic Sea
The Baltic Sea is an arm of the Atlantic Ocean that is enclosed by the countries of Denmark, Estonia, Finland, Germany, Latvia, Lithuania, Poland, Russia, Sweden, and the North European Plain, North and Central European Plain regions. It is the ...
to 4.0% in the Red Sea
The Red Sea is a sea inlet of the Indian Ocean, lying between Africa and Asia. Its connection to the ocean is in the south, through the Bab-el-Mandeb Strait and the Gulf of Aden. To its north lie the Sinai Peninsula, the Gulf of Aqaba, and th ...
. (The Dead Sea
The Dead Sea (; or ; ), also known by #Names, other names, is a landlocked salt lake bordered by Jordan to the east, the Israeli-occupied West Bank to the west and Israel to the southwest. It lies in the endorheic basin of the Jordan Rift Valle ...
, known for its ultra-high salinity levels of between 30 and 40%, is really a salt lake
A salt lake or saline lake is a landlocked body of water that has a concentration of salts (typically sodium chloride) and other dissolved minerals significantly higher than most lakes (often defined as at least three grams of salt per liter). I ...
.)
Tide
Tides are the rise and fall of sea levels caused by the combined effects of the gravitational forces exerted by the Moon (and to a much lesser extent, the Sun) and are also caused by the Earth and Moon orbiting one another.
Tide tables ...
s are the cyclic rising and falling of local sea levels caused by the tidal force
The tidal force or tide-generating force is the difference in gravitational attraction between different points in a gravitational field, causing bodies to be pulled unevenly and as a result are being stretched towards the attraction. It is the ...
s of the Moon and the Sun acting on the oceans. Tides cause changes in the depth of the marine and estuarine
An estuary is a partially enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea. Estuaries form a transition zone between river environments and maritime environm ...
water bodies and produce oscillating currents known as tidal streams. The changing tide produced at a given location is the result of the changing positions of the Moon and Sun relative to the Earth coupled with the effects of Earth rotation and the local bathymetry
Bathymetry (; ) is the study of underwater depth of ocean floors ('' seabed topography''), river floors, or lake floors. In other words, bathymetry is the underwater equivalent to hypsometry or topography. The first recorded evidence of wate ...
. The strip of seashore that is submerged at high tide and exposed at low tide, the intertidal zone
The intertidal zone or foreshore is the area above water level at low tide and underwater at high tide; in other words, it is the part of the littoral zone within the tidal range. This area can include several types of habitats with various ...
, is an important ecological product of ocean tides.
Effects on life
 From a
From a biological
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, origin, evolution, and distribution of ...
standpoint, water has many distinct properties that are critical for the proliferation of life. It carries out this role by allowing organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s to react in ways that ultimately allow replication. All known forms of life depend on water. Water is vital both as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
in which many of the body's solutes dissolve and as an essential part of many metabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
processes within the body. Metabolism is the sum total of anabolism
Anabolism () is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an Endergonic reaction, endergonic process. Anabolism is the building-up aspect of metabo ...
and catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipid ...
. In anabolism, water is removed from molecules (through energy requiring enzymatic chemical reactions) to grow larger molecules (e.g., starches, triglycerides, and proteins for storage of fuels and information). In catabolism, water is used to break bonds to generate smaller molecules (e.g., glucose, fatty acids, and amino acids to be used for fuels for energy use or other purposes). Without water, these particular metabolic processes could not exist.
Water is fundamental to both photosynthesis and respiration. Photosynthetic cells use the sun's energy to split off water's hydrogen from oxygen. In the presence of sunlight, hydrogen is combined with (absorbed from air or water) to form glucose and release oxygen. All living cells use such fuels and oxidize the hydrogen and carbon to capture the sun's energy and reform water and in the process (cellular respiration).
Water is also central to acid-base neutrality and enzyme function. An acid, a hydrogen ion (, that is, a proton) donor, can be neutralized by a base, a proton acceptor such as a hydroxide ion () to form water. Water is considered to be neutral, with a pH (the negative log of the hydrogen ion concentration) of 7 in an ideal state. Acids
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
have pH values less than 7 while bases have values greater than 7.
Aquatic life forms
Earth's surface waters are filled with life. The earliest life forms appeared in water; nearly all fish live exclusively in water, and there are many types of marine mammals, such as dolphins and whales. Some kinds of animals, such as amphibian
Amphibians are ectothermic, anamniote, anamniotic, tetrapod, four-limbed vertebrate animals that constitute the class (biology), class Amphibia. In its broadest sense, it is a paraphyletic group encompassing all Tetrapod, tetrapods, but excl ...
s, spend portions of their lives in water and portions on land. Plants such as kelp
Kelps are large brown algae or seaweeds that make up the order (biology), order Laminariales. There are about 30 different genus, genera. Despite its appearance and use of photosynthesis in chloroplasts, kelp is technically not a plant but a str ...
and algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
grow in the water and are the basis for some underwater ecosystems. Plankton
Plankton are the diverse collection of organisms that drift in Hydrosphere, water (or atmosphere, air) but are unable to actively propel themselves against ocean current, currents (or wind). The individual organisms constituting plankton are ca ...
is generally the foundation of the ocean food chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as ...
.
Aquatic vertebrates must obtain oxygen to survive, and they do so in various ways. Fish have gills
A gill () is a respiratory organ that many aquatic organisms use to extract dissolved oxygen from water and to excrete carbon dioxide. The gills of some species, such as hermit crabs, have adapted to allow respiration on land provided they are ...
instead of lungs
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syste ...
, although some species of fish, such as the lungfish
Lungfish are freshwater vertebrates belonging to the class Dipnoi. Lungfish are best known for retaining ancestral characteristics within the Osteichthyes, including the ability to breathe air, and ancestral structures within Sarcopterygii, inc ...
, have both. Marine mammal
Marine mammals are mammals that rely on marine ecosystems for their existence. They include animals such as cetaceans, pinnipeds, sirenians, sea otters and polar bears. They are an informal group, unified only by their reliance on marine enviro ...
s, such as dolphins, whales, otter
Otters are carnivorous mammals in the subfamily Lutrinae. The 13 extant otter species are all semiaquatic, aquatic, or marine. Lutrinae is a branch of the Mustelidae family, which includes weasels, badgers, mink, and wolverines, among ...
s, and seals need to surface periodically to breathe air. Some amphibians are able to absorb oxygen through their skin. Invertebrates exhibit a wide range of modifications to survive in poorly oxygenated waters including breathing tubes (see insect
Insects (from Latin ') are Hexapoda, hexapod invertebrates of the class (biology), class Insecta. They are the largest group within the arthropod phylum. Insects have a chitinous exoskeleton, a three-part body (Insect morphology#Head, head, ...
and mollusc siphons) and gills
A gill () is a respiratory organ that many aquatic organisms use to extract dissolved oxygen from water and to excrete carbon dioxide. The gills of some species, such as hermit crabs, have adapted to allow respiration on land provided they are ...
(''Carcinus
''Carcinus'' ( '' Karkinos'') is a genus of crabs, which includes '' Carcinus maenas'', an important invasive species, and '' C. aestuarii'', a species endemic to the Mediterranean Sea.
''Carcinus maenas''
''C. maenas'' is among the 100 "wor ...
''). However, as invertebrate life evolved in an aquatic habitat most have little or no specialization for respiration in water.
Effects on human civilization
 Civilization has historically flourished around rivers and major waterways;
Civilization has historically flourished around rivers and major waterways; Mesopotamia
Mesopotamia is a historical region of West Asia situated within the Tigris–Euphrates river system, in the northern part of the Fertile Crescent. Today, Mesopotamia is known as present-day Iraq and forms the eastern geographic boundary of ...
, one of the so-called cradles of civilization
A cradle of civilization is a location and a culture where civilization was developed independent of other civilizations in other locations. A civilization is any complex society characterized by the development of state (polity), the state, ...
, was situated between the major rivers Tigris
The Tigris ( ; see #Etymology, below) is the eastern of the two great rivers that define Mesopotamia, the other being the Euphrates. The river flows south from the mountains of the Armenian Highlands through the Syrian Desert, Syrian and Arabia ...
and Euphrates
The Euphrates ( ; see #Etymology, below) is the longest and one of the most historically important rivers of West Asia. Tigris–Euphrates river system, Together with the Tigris, it is one of the two defining rivers of Mesopotamia (). Originati ...
; the ancient society of the Egyptians
Egyptians (, ; , ; ) are an ethnic group native to the Nile, Nile Valley in Egypt. Egyptian identity is closely tied to Geography of Egypt, geography. The population is concentrated in the Nile Valley, a small strip of cultivable land stretchi ...
depended entirely upon the Nile
The Nile (also known as the Nile River or River Nile) is a major north-flowing river in northeastern Africa. It flows into the Mediterranean Sea. The Nile is the longest river in Africa. It has historically been considered the List of river sy ...
. The early Indus Valley civilization
The Indus Valley Civilisation (IVC), also known as the Indus Civilisation, was a Bronze Age civilisation in the northwestern regions of South Asia, lasting from 3300 BCE to 1300 BCE, and in its mature form from 2600 BCE ...
() developed along the Indus River and tributaries that flowed out of the Himalayas
The Himalayas, or Himalaya ( ), is a mountain range in Asia, separating the plains of the Indian subcontinent from the Tibetan Plateau. The range has some of the Earth's highest peaks, including the highest, Mount Everest. More than list of h ...
. Rome
Rome (Italian language, Italian and , ) is the capital city and most populated (municipality) of Italy. It is also the administrative centre of the Lazio Regions of Italy, region and of the Metropolitan City of Rome. A special named with 2, ...
was also founded on the banks of the Italian river Tiber
The Tiber ( ; ; ) is the List of rivers of Italy, third-longest river in Italy and the longest in Central Italy, rising in the Apennine Mountains in Emilia-Romagna and flowing through Tuscany, Umbria, and Lazio, where it is joined by the R ...
. Large metropolis
A metropolis () is a large city or conurbation which is a significant economic, political, and cultural area for a country or region, and an important hub for regional or international connections, commerce, and communications.
A big city b ...
es like Rotterdam
Rotterdam ( , ; ; ) is the second-largest List of cities in the Netherlands by province, city in the Netherlands after the national capital of Amsterdam. It is in the Provinces of the Netherlands, province of South Holland, part of the North S ...
, London, Montreal, Paris, New York City, Buenos Aires
Buenos Aires, controlled by the government of the Autonomous City of Buenos Aires, is the Capital city, capital and largest city of Argentina. It is located on the southwest of the Río de la Plata. Buenos Aires is classified as an Alpha− glob ...
, Shanghai, Tokyo, Chicago, and Hong Kong owe their success in part to their easy accessibility via water and the resultant expansion of trade. Islands with safe water ports, like Singapore, have flourished for the same reason. In places such as North Africa and the Middle East, where water is more scarce, access to clean drinking water was and is a major factor in human development.
Health and pollution
Water fit for human consumption is called drinking water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
or potable water. Water that is not potable may be made potable by filtration or distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
, or by a range of other methods. More than 660 million people do not have access to safe drinking water.
Water that is not fit for drinking but is not harmful to humans when used for swimming or bathing is called by various names other than potable or drinking water, and is sometimes called safe water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
, or "safe for bathing". Chlorine is a skin and mucous membrane irritant that is used to make water safe for bathing or drinking. Its use is highly technical and is usually monitored by government regulations (typically 1 part per million (ppm) for drinking water, and 1–2 ppm of chlorine not yet reacted with impurities for bathing water). Water for bathing may be maintained in satisfactory microbiological condition using chemical disinfectants such as chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
or ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
or by the use of ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
light.
Water reclamation
Water reclamation is the process of converting municipal wastewater or sewage and industrial wastewater into water that can be reused for a variety of purposes. It is also called wastewater reuse, water reuse or water recycling. There are many ty ...
is the process of converting wastewater (most commonly sewage
Sewage (or domestic sewage, domestic wastewater, municipal wastewater) is a type of wastewater that is produced by a community of people. It is typically transported through a sewerage, sewer system. Sewage consists of wastewater discharged fro ...
, also called municipal wastewater) into water that can be reuse
Reuse is the action or practice of using an item, whether for its original purpose (conventional reuse) or to fulfill a different function (creative reuse or repurposing). It should be distinguished from recycling, which is the breaking down of ...
d for other purposes. There are 2.3 billion people who reside in nations with water scarcities, which means that each individual receives less than of water annually. of municipal wastewater are produced globally each year.hydrologic cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fai ...
, but pressures over access to it result from the naturally uneven distribution in space and time, growing economic demands by agriculture and industry, and rising populations. Currently, nearly a billion people around the world lack access to safe, affordable water. In 2000, the United Nations established the Millennium Development Goals
In the United Nations, the Millennium Development Goals (MDGs) were eight international development goals for the year 2015 created following the Millennium Summit, following the adoption of the United Nations Millennium Declaration. These w ...
for water to halve by 2015 the proportion of people worldwide without access to safe water and sanitation
Sanitation refers to public health conditions related to clean drinking water and treatment and disposal of human excreta and sewage. Preventing human contact with feces is part of sanitation, as is hand washing with soap. Sanitation systems ...
. Progress toward that goal was uneven, and in 2015 the UN committed to the Sustainable Development Goals
The ''2030 Agenda for Sustainable Development'', adopted by all United Nations (UN) members in 2015, created 17 world Sustainable Development Goals (SDGs). The aim of these global goals is "peace and prosperity for people and the planet" – wh ...
of achieving universal access to safe and affordable water and sanitation by 2030. Poor water quality
Water quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through tr ...
and bad sanitation are deadly; some five million deaths a year are caused by water-related diseases. The World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
estimates that safe water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
could prevent 1.4 million child deaths from diarrhea
Diarrhea (American English), also spelled diarrhoea or diarrhœa (British English), is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration d ...
each year.
In developing countries, 90% of all municipal wastewater
Sewage (or domestic sewage, domestic wastewater, municipal wastewater) is a type of wastewater that is produced by a community of people. It is typically transported through a sewer system. Sewage consists of wastewater discharged from residen ...
still goes untreated into local rivers and streams. Some 50 countries, with roughly a third of the world's population, also suffer from medium or high water scarcity
Water scarcity (closely related to water stress or water crisis) is the lack of fresh water resources to meet the standard water demand. There are two types of water scarcity. One is ''physical.'' The other is ''economic water scarcity''. Physic ...
and 17 of these extract more water annually than is recharged through their natural water cycles. The strain not only affects surface freshwater bodies like rivers and lakes, but it also degrades groundwater resources.
Human uses
Agriculture
The most substantial human use of water is for agriculture, including irrigated agriculture, which accounts for as much as 80 to 90 percent of total human water consumption. In the United States, 42% of freshwater withdrawn for use is for irrigation, but the vast majority of water "consumed" (used and not returned to the environment) goes to agriculture.peak water
Peak water is a concept that underlines the growing constraints on the availability, quality, and use of freshwater resources. Peak water was defined in 2010 by Peter Gleick and Meena Palaniappan. They distinguish between peak renewable, peak n ...
. As populations and economies continue to grow, consumption of water-thirsty meat expands, and new demands rise for biofuels or new water-intensive industries, new water challenges are likely.
An assessment of water management in agriculture was conducted in 2007 by the International Water Management Institute
The International Water Management Institute (IWMI) is a non-profit international water management research organisation under the One CGIAR with its headquarters in Colombo, Sri Lanka, and offices across Africa and Asia. One CGIAR is a reformulat ...
in Sri Lanka to see if the world had sufficient water to provide food for its growing population. It assessed the current availability of water for agriculture on a global scale and mapped out locations suffering from water scarcity. It found that a fifth of the world's people, more than 1.2 billion, live in areas of physical water scarcity, where there is not enough water to meet all demands. A further 1.6 billion people live in areas experiencing economic water scarcity, where the lack of investment in water or insufficient human capacity make it impossible for authorities to satisfy the demand for water. The report found that it would be possible to produce the food required in the future, but that continuation of today's food production and environmental trends would lead to crises in many parts of the world. To avoid a global water crisis, farmers will have to strive to increase productivity to meet growing demands for food, while industries and cities find ways to use water more efficiently.
Water scarcity is also caused by production of water intensive products. For example, cotton: 1 kg of cotton—equivalent of a pair of jeans—requires water to produce. While cotton accounts for 2.4% of world water use, the water is consumed in regions that are already at a risk of water shortage. Significant environmental damage has been caused: for example, the diversion of water by the former Soviet Union
The Union of Soviet Socialist Republics. (USSR), commonly known as the Soviet Union, was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 until Dissolution of the Soviet ...
from the Amu Darya
The Amu Darya ( ),() also shortened to Amu and historically known as the Oxus ( ), is a major river in Central Asia, which flows through Tajikistan, Turkmenistan, Uzbekistan, and Afghanistan. Rising in the Pamir Mountains, north of the Hindu Ku ...
and Syr Darya
The Syr Darya ( ),; ; ; ; ; /. historically known as the Jaxartes ( , ), is a river in Central Asia. The name, which is Persian language, Persian, literally means ''Syr Sea'' or ''Syr River''. It originates in the Tian Shan, Tian Shan Mountain ...
rivers to produce cotton was largely responsible for the disappearance of the Aral Sea
The Aral Sea () was an endorheic lake lying between Kazakhstan to its north and Uzbekistan to its south, which began shrinking in the 1960s and had largely dried up into desert by the 2010s. It was in the Aktobe and Kyzylorda regions of Kazakhst ...
.
File:Water requirement per tonne of food product, OWID.svg, Water requirement per tonne of food product
File:Subsurface drip emission on loamy soil.ogv, Water distribution in subsurface drip irrigation
Drip irrigation or trickle irrigation is a type of micro-irrigation system that has the potential to save water and nutrients by allowing water to drip slowly to the roots of plants, either from above the soil surface or buried below the surfac ...
File:SiphonTubes.JPG, Irrigation
Irrigation (also referred to as watering of plants) is the practice of applying controlled amounts of water to land to help grow crops, landscape plants, and lawns. Irrigation has been a key aspect of agriculture for over 5,000 years and has bee ...
of field crops
As a scientific standard
On 7 April 1795, the gram was defined in France to be equal to "the absolute weight of a volume of pure water equal to a cube of one-hundredth of a meter, and at the temperature of melting ice". For practical purposes though, a metallic reference standard was required, one thousand times more massive, the kilogram. Work was therefore commissioned to determine precisely the mass of one liter of water. In spite of the fact that the decreed definition of the gram specified water at —a highly reproducible ''temperature''—the scientists chose to redefine the standard and to perform their measurements at the temperature of highest water ''density'', which was measured at the time as .
The Kelvin temperature scale
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
of the SI system was based on the triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
of water, defined as exactly , but as of May 2019 is based on the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
instead. The scale is an absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
scale with the same increment as the Celsius temperature scale, which was originally defined according to the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
(set to ) and melting point (set to ) of water.
Natural water consists mainly of the isotopes hydrogen-1 and oxygen-16, but there is also a small quantity of heavier isotopes oxygen-18, oxygen-17, and hydrogen-2 (deuterium). The percentage of the heavier isotopes is very small, but it still affects the properties of water. Water from rivers and lakes tends to contain less heavy isotopes than seawater. Therefore, standard water is defined in the Vienna Standard Mean Ocean Water specification.
For drinking

 The human body contains from 55% to 78% water, depending on body size. To function properly, the body requires between of water per day to avoid dehydration; the precise amount depends on the level of activity, temperature, humidity, and other factors. Most of this is ingested through foods or beverages other than drinking straight water. It is not clear how much water intake is needed by healthy people, though the British Dietetic Association advises that 2.5 liters of total water daily is the minimum to maintain proper hydration, including 1.8 liters (6 to 7 glasses) obtained directly from beverages. Medical literature favors a lower consumption, typically 1 liter of water for an average male, excluding extra requirements due to fluid loss from exercise or warm weather.
The human body contains from 55% to 78% water, depending on body size. To function properly, the body requires between of water per day to avoid dehydration; the precise amount depends on the level of activity, temperature, humidity, and other factors. Most of this is ingested through foods or beverages other than drinking straight water. It is not clear how much water intake is needed by healthy people, though the British Dietetic Association advises that 2.5 liters of total water daily is the minimum to maintain proper hydration, including 1.8 liters (6 to 7 glasses) obtained directly from beverages. Medical literature favors a lower consumption, typically 1 liter of water for an average male, excluding extra requirements due to fluid loss from exercise or warm weather. An original recommendation for water intake in 1945 by the Food and Nutrition Board of the U.S. National Research Council read: "An ordinary standard for diverse persons is 1 milliliter for each calorie of food. Most of this quantity is contained in prepared foods." The latest dietary reference intake report by the U.S. National Research Council in general recommended, based on the median total water intake from US survey data (including food sources): for men and of water total for women, noting that water contained in food provided approximately 19% of total water intake in the survey.
Specifically, pregnant and breastfeeding women need additional fluids to stay hydrated. The US Institute of Medicine recommends that, on average, men consume and women ; pregnant women should increase intake to and breastfeeding women should get 3 liters (12 cups), since an especially large amount of fluid is lost during nursing. Also noted is that normally, about 20% of water intake comes from food, while the rest comes from drinking water and beverages (Caffeine, caffeinated included). Water is excreted from the body in multiple forms; through urine and feces, through sweating, and by exhalation of water vapor in the breath. With physical exertion and heat exposure, water loss will increase and daily fluid needs may increase as well.
Humans require water with few impurities. Common impurities include metal salts and oxides, including copper, iron, calcium and lead, and harmful bacteria, such as ''Vibrio''. Some solutes are acceptable and even desirable for taste enhancement and to provide needed electrolytes.
The single largest (by volume) freshwater resource suitable for drinking is Lake Baikal in Siberia.
An original recommendation for water intake in 1945 by the Food and Nutrition Board of the U.S. National Research Council read: "An ordinary standard for diverse persons is 1 milliliter for each calorie of food. Most of this quantity is contained in prepared foods." The latest dietary reference intake report by the U.S. National Research Council in general recommended, based on the median total water intake from US survey data (including food sources): for men and of water total for women, noting that water contained in food provided approximately 19% of total water intake in the survey.
Specifically, pregnant and breastfeeding women need additional fluids to stay hydrated. The US Institute of Medicine recommends that, on average, men consume and women ; pregnant women should increase intake to and breastfeeding women should get 3 liters (12 cups), since an especially large amount of fluid is lost during nursing. Also noted is that normally, about 20% of water intake comes from food, while the rest comes from drinking water and beverages (Caffeine, caffeinated included). Water is excreted from the body in multiple forms; through urine and feces, through sweating, and by exhalation of water vapor in the breath. With physical exertion and heat exposure, water loss will increase and daily fluid needs may increase as well.
Humans require water with few impurities. Common impurities include metal salts and oxides, including copper, iron, calcium and lead, and harmful bacteria, such as ''Vibrio''. Some solutes are acceptable and even desirable for taste enhancement and to provide needed electrolytes.
The single largest (by volume) freshwater resource suitable for drinking is Lake Baikal in Siberia.
Washing
Transportation
Chemical uses
Water is widely used in chemical reactions as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
or reactant and less commonly as a solute or catalyst. In inorganic reactions, water is a common solvent, dissolving many ionic compounds, as well as other polar compounds such as ammonia and Hydrogen chalcogenide, compounds closely related to water. In organic reactions, it is not usually used as a reaction solvent, because it does not dissolve the reactants well and is amphoteric (acidic ''and'' basic) and nucleophilic. Nevertheless, these properties are sometimes desirable. Also, acceleration of Diels-Alder reactions by water has been observed. Supercritical water has recently been a topic of research. Oxygen-saturated supercritical water combusts organic pollutants efficiently.
Heat exchange
Water and steam are a common fluid used for heat exchanger, heat exchange, due to its availability and high Heat capacity of water, heat capacity, both for cooling and heating. Cool water may even be naturally available from a lake or the sea. It is especially effective to transport heat through vaporization
Vaporization (or vapo(u)risation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenome ...
and condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of water because of its large latent heat of vaporization. A disadvantage is that metals commonly found in industries such as steel and copper are oxidation, oxidized faster by untreated water and steam. In almost all thermal power stations, water is used as the working fluid (used in a closed-loop between boiler, steam turbine, and condenser), and the coolant (used to exchange the waste heat to a water body or carry it away by evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
in a cooling tower). In the United States, cooling power plants is the largest use of water.["Water Use in the United States"](_blank)
''National Atlas''.
In the nuclear power industry, water can also be used as a neutron moderator. In most nuclear reactors, water is both a coolant and a moderator. This provides something of a passive safety measure, as removing the water from the reactor also void coefficient, slows the nuclear reaction down. However other methods are favored for stopping a reaction and it is preferred to keep the nuclear core covered with water so as to ensure adequate cooling.
Fire considerations
 Water has a high heat of vaporization and is relatively inert, which makes it a good Fire fighting#Use of water, fire extinguishing fluid. The evaporation of water carries heat away from the fire. It is dangerous to use water on fires involving oils and organic solvents because many organic materials float on water and the water tends to spread the burning liquid.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot carbon such as coal, charcoal, or coke (fuel), coke graphite, decompose the water, producing water gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved in this case did not come from fire-fighting but from the reactor's own water cooling system. A steam explosion occurred when the extreme overheating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of a reaction between steam and hot zirconium.
Some metallic oxides, most notably those of
Water has a high heat of vaporization and is relatively inert, which makes it a good Fire fighting#Use of water, fire extinguishing fluid. The evaporation of water carries heat away from the fire. It is dangerous to use water on fires involving oils and organic solvents because many organic materials float on water and the water tends to spread the burning liquid.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot carbon such as coal, charcoal, or coke (fuel), coke graphite, decompose the water, producing water gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved in this case did not come from fire-fighting but from the reactor's own water cooling system. A steam explosion occurred when the extreme overheating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of a reaction between steam and hot zirconium.
Some metallic oxides, most notably those of alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and alkaline earth metals
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are a ...
, produce so much heat in reaction with water that a fire hazard can develop. The alkaline earth oxide Calcium oxide, quicklime, also known as calcium oxide, is a mass-produced substance that is often transported in paper bags. If these are soaked through, they may ignite as their contents react with water.
Recreation
 Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating,
Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating, surfing
Surfing is a surface water sport in which an individual, a surfer (or two in tandem surfing), uses a board to ride on the forward section, or face, of a moving wave of water, which usually carries the surfer towards the shore. Waves suita ...
and Underwater diving, diving. In addition, some sports, like ice hockey and ice skating
Ice skating is the Human-powered transport, self-propulsion and gliding of a person across an ice surface, using metal-bladed ice skates. People skate for various reasons, including recreation (fun), exercise, competitive sports, and commuting. ...
, are played on ice. Lakesides, beaches and water parks are popular places for people to go to relax and enjoy recreation. Many find the sound and appearance of flowing water to be calming, and fountains and other flowing water structures are popular decorations. Some keep fish and other flora and fauna inside aquariums or ponds for show, fun, and companionship. Humans also use water for snow sports such as skiing
Skiing is the use of skis to glide on snow for basic transport, a recreational activity, or a competitive winter sport. Many types of competitive skiing events are recognized by the International Olympic Committee (IOC), and the International S ...
, sledding, snowmobiling or snowboarding
Snowboarding is a recreational and competitive activity that involves descending a snow-covered surface while standing on a snowboard that is almost always attached to a rider's feet. It features in the Winter Olympic Games and Winter Paralym ...
, which require the water to be at a low temperature either as ice or crystallized into snow.
Water industry
The water industry provides drinking water and wastewater services (including sewage treatment) to households and industry. Water supply facilities include water wells, cisterns for rainwater harvesting, water supply networks, and water purification facilities, water tanks, water towers, water pipes including old Aqueduct (watercourse), aqueducts. Atmospheric water generators are in development.
Drinking water is often collected at spring (hydrosphere), springs, extracted from artificial Boring (earth), borings (wells) in the ground, or pumped from lakes and rivers. Building more wells in adequate places is thus a possible way to produce more water, assuming the aquifers can supply an adequate flow. Other water sources include rainwater collection. Water may require purification for human consumption. This may involve the removal of undissolved substances, dissolved substances and harmful microbes. Popular methods are filter (water), filtering with sand which only removes undissolved material, while Water chlorination, chlorination and boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor, vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to ...
kill harmful microbes. Distillation does all three functions. More advanced techniques exist, such as reverse osmosis. Desalination of abundant seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
is a more expensive solution used in coastal arid climate
Climate is the long-term weather pattern in a region, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteoro ...
s.
The distribution of drinking water is done through municipal water systems, tanker delivery or as bottled water. Governments in many countries have programs to distribute water to the needy at no charge.
Reducing usage by using drinking (potable) water only for human consumption is another option. In some cities such as Hong Kong, seawater is extensively used for flushing toilets citywide to Water conservation, conserve freshwater resources.
Water pollution, Polluting water may be the biggest single misuse of water; to the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as externality, externalities for which the market cannot account. Thus other people pay the price of water pollution, while the private firms' profits are not redistributed to the local population, victims of this pollution. Pharmaceuticals consumed by humans often end up in the waterways and can have detrimental effects on marine biology, aquatic life if they bioaccumulation, bioaccumulate and if they are not biodegradable.
Municipal and industrial wastewater treatment, industrial wastewater are typically treated at wastewater treatment plants. Mitigation of polluted surface runoff is addressed through a variety of Surface runoff#Mitigation and treatment, prevention and treatment techniques.
Industrial applications
Many industrial processes rely on reactions using chemicals dissolved in water, suspension of solids in water slurry, slurries or using water to dissolve and extract substances, or to wash products or process equipment. Processes such as mining, chemical pulping, pulp bleaching, paper manufacturing, textile production, dyeing, printing, and cooling of power plants use large amounts of water, requiring a dedicated water source, and often cause significant water pollution.
Water is used in power generation. Hydroelectricity is electricity obtained from hydropower. Hydroelectric power comes from water driving a water turbine connected to a generator. Hydroelectricity is a low-cost, non-polluting, renewable energy source. The energy is supplied by the motion of water. Typically a dam is constructed on a river, creating an artificial lake behind it. Water flowing out of the lake is forced through turbines that turn generators.
Pressurized water is used in Hydrodemolition, water blasting and water jet cutters. High pressure water guns are used for precise cutting. It works very well, is relatively safe, and is not harmful to the environment. It is also used in the cooling of machinery to prevent overheating, or prevent saw blades from overheating.
Water is also used in many industrial processes and machines, such as the steam turbine and heat exchanger, in addition to its use as a chemical solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
. Discharge of untreated water from industrial uses is water pollution, pollution. Pollution includes discharged solutes (chemical pollution) and discharged coolant water (thermal pollution). Industry requires pure water for many applications and uses a variety of purification techniques both in water supply and discharge.
Food processing

 Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used for dishwashing. Water also plays many critical roles within the field of food science.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure that occurs at higher elevations. One mole (unit), mole of sucrose (sugar) per kilogram of water raises the boiling point of water by , and one mole of salt per kg raises the boiling point by ; similarly, increasing the number of dissolved particles lowers water's freezing point.
Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used for dishwashing. Water also plays many critical roles within the field of food science.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure that occurs at higher elevations. One mole (unit), mole of sucrose (sugar) per kilogram of water raises the boiling point of water by , and one mole of salt per kg raises the boiling point by ; similarly, increasing the number of dissolved particles lowers water's freezing point.
Medical use
Water for injection is on the World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
's World Health Organization's list of essential medicines, list of essential medicines.
Distribution in nature
In the universe
 Much of the universe's water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing "140 trillion times more water than all of Earth's oceans combined" around a quasar located 12 billion light years from Earth. According to the researchers, the "discovery shows that water has been prevalent in the universe for nearly its entire existence".
Much of the universe's water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing "140 trillion times more water than all of Earth's oceans combined" around a quasar located 12 billion light years from Earth. According to the researchers, the "discovery shows that water has been prevalent in the universe for nearly its entire existence".
Water vapor
Water is present as vapor in:
* Solar atmosphere, Atmosphere of the Sun: in detectable trace amounts[Water Found in Extrasolar Planet's Atmosphere](_blank)
– Space.com Tau Boötis b, HAT-P-11b,[Andersson, Jonas (June 2012)]
Water in stellar atmospheres "Is a novel picture required to explain the atmospheric behavior of water in red giant stars?"
Lund Observatory, Lund University, Sweden
* Circumstellar disks: including those of more than half of T Tauri stars such as AA Tauri
Liquid water
Liquid water is present on Earth, covering 71% of its surface.
Water ice
Water is present as ice on:
*  Water on Mars, Mars: under the regolith and at the poles.
* Earth–Moon system: mainly as ice sheets on Earth and in Lunar craters and volcanic rocks NASA reported the detection of water molecules by NASA's Moon Mineralogy Mapper aboard the Indian Space Research Organization's Chandrayaan-1 spacecraft in September 2009.
* Ceres (dwarf planet), Ceres
Water on Mars, Mars: under the regolith and at the poles.
* Earth–Moon system: mainly as ice sheets on Earth and in Lunar craters and volcanic rocks NASA reported the detection of water molecules by NASA's Moon Mineralogy Mapper aboard the Indian Space Research Organization's Chandrayaan-1 spacecraft in September 2009.
* Ceres (dwarf planet), Ceres[ (supporting online material, table S1)]
* Pluto–Charon (moon), Charon system
Exotic forms
Water and other Volatile (astrogeology), volatiles probably comprise much of the internal structures of Uranus and Neptune and the water in the deeper layers may be in the form of ionic water in which the molecules break down into a soup of hydrogen and oxygen ions, and deeper still as superionic water in which the oxygen crystallizes, but the hydrogen ions float about freely within the oxygen lattice.[Weird water lurking inside giant planets](_blank)
, ''New Scientist'', 1 September 2010, Magazine issue 2776.
Water and planetary habitability
The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth are vital to the existence of Organism, life on Earth as we know it. The Earth is located in the habitable zone of the Solar System; if it were slightly closer to or farther from the Sun (about 5%, or about 8 million kilometers), the conditions which allow the three forms to be present simultaneously would be far less likely to exist.
Earth's gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force b ...
allows it to hold an Celestial body atmosphere, atmosphere. Water vapor and carbon dioxide in the atmosphere provide a temperature buffer (greenhouse effect) which helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would allow temperature extremes, thus preventing the accumulation of water except in polar ice caps (as on Mars).
The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.
The state of water on a planet depends on ambient pressure, which is determined by the planet's gravity. If a planet is sufficiently massive, the water on it may be solid even at high temperatures, because of the high pressure caused by gravity, as it was observed on exoplanets Gliese 436 b and GJ 1214 b.
Law, politics, and crisis
 Water politics is politics affected by water and water resources. Water, particularly fresh water, is a strategic resource across the world and an important element in many political conflicts. It causes health impacts and damage to biodiversity.
Access to safe drinking water has improved over the last decades in almost every part of the world, but approximately one billion people still lack access to safe water and over 2.5 billion lack access to adequate
Water politics is politics affected by water and water resources. Water, particularly fresh water, is a strategic resource across the world and an important element in many political conflicts. It causes health impacts and damage to biodiversity.
Access to safe drinking water has improved over the last decades in almost every part of the world, but approximately one billion people still lack access to safe water and over 2.5 billion lack access to adequate sanitation
Sanitation refers to public health conditions related to clean drinking water and treatment and disposal of human excreta and sewage. Preventing human contact with feces is part of sanitation, as is hand washing with soap. Sanitation systems ...
.International Water Management Institute
The International Water Management Institute (IWMI) is a non-profit international water management research organisation under the One CGIAR with its headquarters in Colombo, Sri Lanka, and offices across Africa and Asia. One CGIAR is a reformulat ...
of policies in six countries that rely on the Mekong river for water found that thorough and transparent cost-benefit analyses and environmental impact assessments were rarely undertaken. They also discovered that Cambodia's draft water law was much more complex than it needed to be.
In 2004, the UK charity WaterAid reported that a child dies every 15 seconds from easily preventable water-related diseases, which are often tied to a lack of adequate sanitation.drinking water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
and 3.6 billion (46%) lack access to safely managed sanitation. People in urban areas (2.4 billion) will face water scarcity
Water scarcity (closely related to water stress or water crisis) is the lack of fresh water resources to meet the standard water demand. There are two types of water scarcity. One is ''physical.'' The other is ''economic water scarcity''. Physic ...
by 2050.International Water Management Institute
The International Water Management Institute (IWMI) is a non-profit international water management research organisation under the One CGIAR with its headquarters in Colombo, Sri Lanka, and offices across Africa and Asia. One CGIAR is a reformulat ...
undertakes projects with the aim of using effective water management to reduce poverty. Water related conventions are United Nations Convention to Combat Desertification (UNCCD), International Convention for the Prevention of Pollution from Ships, United Nations Convention on the Law of the Sea and Ramsar Convention. World Day for Water takes place on 22 March and World Oceans Day on 8 June.
In culture
Religion
 Water is considered a purifier in most religions. Faiths that incorporate ritual washing (Ritual purification, ablution) include Christianity, Hinduism, Islam, Judaism, the Rastafari movement, Shinto, Taoism, and Wicca. Immersion (or aspersion or affusion) of a person in water is a central Sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Islam (''Ghusl''), Judaism (''mikvah'') and Sikhism (''Amrit Sanskar''). In addition, a ritual bath in pure water is performed for the dead in many religions including Islam and Judaism. In Islam, the five daily prayers can be done in most cases after washing certain parts of the body using clean water (''wudu''), unless water is unavailable (see ''Tayammum''). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of ''misogi'').
In Christianity, holy water is water that has been sanctified by a priest for the purpose of baptism, the Blessing (Roman Catholic Church), blessing of persons, places, and objects, or as a means of repelling evil.
In Zoroastrianism, water (''aban, āb'') is respected as the source of life.
Water is considered a purifier in most religions. Faiths that incorporate ritual washing (Ritual purification, ablution) include Christianity, Hinduism, Islam, Judaism, the Rastafari movement, Shinto, Taoism, and Wicca. Immersion (or aspersion or affusion) of a person in water is a central Sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Islam (''Ghusl''), Judaism (''mikvah'') and Sikhism (''Amrit Sanskar''). In addition, a ritual bath in pure water is performed for the dead in many religions including Islam and Judaism. In Islam, the five daily prayers can be done in most cases after washing certain parts of the body using clean water (''wudu''), unless water is unavailable (see ''Tayammum''). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of ''misogi'').
In Christianity, holy water is water that has been sanctified by a priest for the purpose of baptism, the Blessing (Roman Catholic Church), blessing of persons, places, and objects, or as a means of repelling evil.
In Zoroastrianism, water (''aban, āb'') is respected as the source of life.
Philosophy
 The Ancient Greek philosopher Empedocles saw Water (classical element), water as one of the four classical elements (along with fire, earth, and Air (classical element), air), and regarded it as an ylem, or basic substance of the universe. Thales, whom Aristotle portrayed as an astronomer and an engineer, theorized that the earth, which is denser than water, emerged from the water. Thales, a monist, believed further that all things are made from water. Plato believed that the shape of water is an icosahedron – flowing easily compared to the cube-shaped earth.
The theory of the Humorism, four bodily humors associated water with phlegm, as being cold and moist. The Water (classical element), classical element of water was also one of the Five elements (Chinese philosophy), five elements in traditional Chinese philosophy (along with earth (classical element), earth, fire (classical element), fire, wood (classical element), wood, and metal (classical element), metal).
Some traditional and popular Asian philosophy, Asian philosophical systems take water as a role-model. James Legge's 1891 translation of the ''Dao De Jing'' states, "The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao" and "There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed." ''Guanzi (text), Guanzi'' in the "Shui di" 水地 chapter further elaborates on the symbolism of water, proclaiming that "man is water" and attributing natural qualities of the people of different Chinese regions to the character of local water resources.
The Ancient Greek philosopher Empedocles saw Water (classical element), water as one of the four classical elements (along with fire, earth, and Air (classical element), air), and regarded it as an ylem, or basic substance of the universe. Thales, whom Aristotle portrayed as an astronomer and an engineer, theorized that the earth, which is denser than water, emerged from the water. Thales, a monist, believed further that all things are made from water. Plato believed that the shape of water is an icosahedron – flowing easily compared to the cube-shaped earth.
The theory of the Humorism, four bodily humors associated water with phlegm, as being cold and moist. The Water (classical element), classical element of water was also one of the Five elements (Chinese philosophy), five elements in traditional Chinese philosophy (along with earth (classical element), earth, fire (classical element), fire, wood (classical element), wood, and metal (classical element), metal).
Some traditional and popular Asian philosophy, Asian philosophical systems take water as a role-model. James Legge's 1891 translation of the ''Dao De Jing'' states, "The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao" and "There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed." ''Guanzi (text), Guanzi'' in the "Shui di" 水地 chapter further elaborates on the symbolism of water, proclaiming that "man is water" and attributing natural qualities of the people of different Chinese regions to the character of local water resources.
Folklore
"Living water" features in Germanic and Slavic Folklore, folktales as a means of bringing the dead back to life. Note the Grimms' Fairy Tales, Grimm fairy-tale ("The Water of Life (German fairy tale), The Water of Life") and the Russian dichotomy of and . The Fountain of Youth represents a related concept of Magic (supernatural), magical waters allegedly preventing aging.
Art and activism
In the significant Modernist literature, modernist novel ''Ulysses (novel), Ulysses'' (1922) by Irish writer James Joyce, the chapter "Ithaca" takes the form of a catechism of 309 questions and answers, one of which is known as the "water hymn".
Dihydrogen monoxide parody
'Dihydrogen monoxide' is a technically correct but rarely used chemical name of water. This name has been used in a series of hoaxes and pranks that mock scientific illiteracy. This began in 1983, when an April Fools' Day article appeared in a newspaper in Durand, Michigan. The false story consisted of safety concerns about the substance.
Music
The word "Water" has been used by many Florida based Rapping, rappers as a sort of catchphrase or adlib. Rappers who have done this include BLP Kosher and Ski Mask the Slump God. To go even further some rappers have made whole songs dedicated to the water in Florida, such as the 2023 Danny Towers song "Florida Water".
See also
*
* is a collection of the chemical and physical properties of water.
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Notes
References
Works cited
*
*
*
*
Further reading
* Debenedetti, PG., and HE Stanley, "Supercooled and Glassy Water", ''Physics Today'' 56 (6), pp. 40–46 (2003)
Downloadable PDF (1.9 MB)
* Gleick, PH., (editor), ''The World's Water: The Biennial Report on Freshwater Resources''. Island Press, Washington, D.C. (published every two years, beginning in 1998.
The World's Water, Island Press
*
Journal of Contemporary Water Research & Education
* Postel, S., ''Last Oasis: Facing Water Scarcity''. W.W. Norton and Company, New York. 1992
* Reisner, M., ''Cadillac Desert: The American West and Its Disappearing Water''. Penguin Books, New York. 1986.
United Nations World Water Development Report
. Produced every three years.
* St. Fleur, Nicholas
. "The water you drink is older than the planet you're standing on." ''The New York Times'' (15 April 2016)
External links
The World's Water Data Page
FAO Comprehensive Water Database, AQUASTAT
Water science school
(USGS)
Portal to The World Bank's strategy, work and associated publications on water resources
America Water Resources Association
Water on the web
Water structure and science
"Why water is one of the weirdest things in the universe"
''Ideas'', BBC, Video, 3:16 minutes, 2019
The chemistry of water
(NSF special report)
* [https://www.pbs.org/wgbh/molecule-that-made-us/ ''H2O: The Molecule That Made Us''], a 2020 PBS documentary
{{DEFAULTSORT:Water
Water,
Articles containing video clips
Hydrogen compounds
Triatomic molecules
Inorganic solvents
Liquids
Materials that expand upon freezing
Nuclear reactor coolants
Oxides
Oxygen compounds
 In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are '' lone pairs'' of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms. The O–H bond length is about 0.096 nm.
Other substances have a tetrahedral molecular structure, for example
In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are '' lone pairs'' of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms. The O–H bond length is about 0.096 nm.
Other substances have a tetrahedral molecular structure, for example 
 The water cycle (known scientifically as the hydrologic cycle) is the continuous exchange of water within the
The water cycle (known scientifically as the hydrologic cycle) is the continuous exchange of water within the  Civilization has historically flourished around rivers and major waterways;
Civilization has historically flourished around rivers and major waterways; 
 Water has a high heat of vaporization and is relatively inert, which makes it a good Fire fighting#Use of water, fire extinguishing fluid. The evaporation of water carries heat away from the fire. It is dangerous to use water on fires involving oils and organic solvents because many organic materials float on water and the water tends to spread the burning liquid.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot carbon such as coal, charcoal, or coke (fuel), coke graphite, decompose the water, producing water gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved in this case did not come from fire-fighting but from the reactor's own water cooling system. A steam explosion occurred when the extreme overheating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of a reaction between steam and hot zirconium.
Some metallic oxides, most notably those of
Water has a high heat of vaporization and is relatively inert, which makes it a good Fire fighting#Use of water, fire extinguishing fluid. The evaporation of water carries heat away from the fire. It is dangerous to use water on fires involving oils and organic solvents because many organic materials float on water and the water tends to spread the burning liquid.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot carbon such as coal, charcoal, or coke (fuel), coke graphite, decompose the water, producing water gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved in this case did not come from fire-fighting but from the reactor's own water cooling system. A steam explosion occurred when the extreme overheating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of a reaction between steam and hot zirconium.
Some metallic oxides, most notably those of  Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating,
Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating, 
 Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used for dishwashing. Water also plays many critical roles within the field of food science.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure that occurs at higher elevations. One mole (unit), mole of sucrose (sugar) per kilogram of water raises the boiling point of water by , and one mole of salt per kg raises the boiling point by ; similarly, increasing the number of dissolved particles lowers water's freezing point.
Solutes in water also affect water activity that affects many chemical reactions and the growth of microbes in food. Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water. Solutes in water lower water activity—this is important to know because most bacterial growth ceases at low levels of water activity. Not only does microbial growth affect the safety of food, but also the preservation and shelf life of food.
Water hardness is also a critical factor in food processing and may be altered or treated by using a chemical ion exchange system. It can dramatically affect the quality of a product, as well as playing a role in sanitation. Water hardness is classified based on concentration of calcium carbonate the water contains. Water is classified as soft if it contains less than 100 mg/L (UK) or less than 60 mg/L (US).
According to a report published by the Water Footprint organization in 2010, a single kilogram of beef requires of water; however, the authors also make clear that this is a global average and circumstantial factors determine the amount of water used in beef production.
Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used for dishwashing. Water also plays many critical roles within the field of food science.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure that occurs at higher elevations. One mole (unit), mole of sucrose (sugar) per kilogram of water raises the boiling point of water by , and one mole of salt per kg raises the boiling point by ; similarly, increasing the number of dissolved particles lowers water's freezing point.
Solutes in water also affect water activity that affects many chemical reactions and the growth of microbes in food. Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water. Solutes in water lower water activity—this is important to know because most bacterial growth ceases at low levels of water activity. Not only does microbial growth affect the safety of food, but also the preservation and shelf life of food.
Water hardness is also a critical factor in food processing and may be altered or treated by using a chemical ion exchange system. It can dramatically affect the quality of a product, as well as playing a role in sanitation. Water hardness is classified based on concentration of calcium carbonate the water contains. Water is classified as soft if it contains less than 100 mg/L (UK) or less than 60 mg/L (US).
According to a report published by the Water Footprint organization in 2010, a single kilogram of beef requires of water; however, the authors also make clear that this is a global average and circumstantial factors determine the amount of water used in beef production.
 Much of the universe's water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing "140 trillion times more water than all of Earth's oceans combined" around a quasar located 12 billion light years from Earth. According to the researchers, the "discovery shows that water has been prevalent in the universe for nearly its entire existence".
Water has been detected in interstellar clouds within the Milky Way. Water probably exists in abundance in other galaxies, too, because its components, hydrogen, and oxygen, are among the most abundant elements in the universe. Based on models of the formation and evolution of the Solar System and that of other star systems, most other planetary systems are likely to have similar ingredients.
Much of the universe's water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing "140 trillion times more water than all of Earth's oceans combined" around a quasar located 12 billion light years from Earth. According to the researchers, the "discovery shows that water has been prevalent in the universe for nearly its entire existence".
Water has been detected in interstellar clouds within the Milky Way. Water probably exists in abundance in other galaxies, too, because its components, hydrogen, and oxygen, are among the most abundant elements in the universe. Based on models of the formation and evolution of the Solar System and that of other star systems, most other planetary systems are likely to have similar ingredients.
 Water on Mars, Mars: under the regolith and at the poles.
* Earth–Moon system: mainly as ice sheets on Earth and in Lunar craters and volcanic rocks NASA reported the detection of water molecules by NASA's Moon Mineralogy Mapper aboard the Indian Space Research Organization's Chandrayaan-1 spacecraft in September 2009.
* Ceres (dwarf planet), Ceres
* Jupiter's moons: Europa (moon), Europa's surface and also that of Ganymede (moon), Ganymede and Callisto (moon), Callisto
* Saturn: in the Rings of Saturn, planet's ring system and on the surface and mantle of Titan (moon), Titan and Enceladus (moon), Enceladus (supporting online material, table S1)
* Pluto–Charon (moon), Charon system
* Comets and other related Kuiper belt and Oort cloud objects
And is also likely present on:
* Mercury (planet), Mercury's poles
* Tethys (moon), Tethys
Water on Mars, Mars: under the regolith and at the poles.
* Earth–Moon system: mainly as ice sheets on Earth and in Lunar craters and volcanic rocks NASA reported the detection of water molecules by NASA's Moon Mineralogy Mapper aboard the Indian Space Research Organization's Chandrayaan-1 spacecraft in September 2009.
* Ceres (dwarf planet), Ceres
* Jupiter's moons: Europa (moon), Europa's surface and also that of Ganymede (moon), Ganymede and Callisto (moon), Callisto
* Saturn: in the Rings of Saturn, planet's ring system and on the surface and mantle of Titan (moon), Titan and Enceladus (moon), Enceladus (supporting online material, table S1)
* Pluto–Charon (moon), Charon system
* Comets and other related Kuiper belt and Oort cloud objects
And is also likely present on:
* Mercury (planet), Mercury's poles
* Tethys (moon), Tethys
 Water is considered a purifier in most religions. Faiths that incorporate ritual washing (Ritual purification, ablution) include Christianity, Hinduism, Islam, Judaism, the Rastafari movement, Shinto, Taoism, and Wicca. Immersion (or aspersion or affusion) of a person in water is a central Sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Islam (''Ghusl''), Judaism (''mikvah'') and Sikhism (''Amrit Sanskar''). In addition, a ritual bath in pure water is performed for the dead in many religions including Islam and Judaism. In Islam, the five daily prayers can be done in most cases after washing certain parts of the body using clean water (''wudu''), unless water is unavailable (see ''Tayammum''). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of ''misogi'').
In Christianity, holy water is water that has been sanctified by a priest for the purpose of baptism, the Blessing (Roman Catholic Church), blessing of persons, places, and objects, or as a means of repelling evil.
In Zoroastrianism, water (''aban, āb'') is respected as the source of life.
Water is considered a purifier in most religions. Faiths that incorporate ritual washing (Ritual purification, ablution) include Christianity, Hinduism, Islam, Judaism, the Rastafari movement, Shinto, Taoism, and Wicca. Immersion (or aspersion or affusion) of a person in water is a central Sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Islam (''Ghusl''), Judaism (''mikvah'') and Sikhism (''Amrit Sanskar''). In addition, a ritual bath in pure water is performed for the dead in many religions including Islam and Judaism. In Islam, the five daily prayers can be done in most cases after washing certain parts of the body using clean water (''wudu''), unless water is unavailable (see ''Tayammum''). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of ''misogi'').
In Christianity, holy water is water that has been sanctified by a priest for the purpose of baptism, the Blessing (Roman Catholic Church), blessing of persons, places, and objects, or as a means of repelling evil.
In Zoroastrianism, water (''aban, āb'') is respected as the source of life.
 The Ancient Greek philosopher Empedocles saw Water (classical element), water as one of the four classical elements (along with fire, earth, and Air (classical element), air), and regarded it as an ylem, or basic substance of the universe. Thales, whom Aristotle portrayed as an astronomer and an engineer, theorized that the earth, which is denser than water, emerged from the water. Thales, a monist, believed further that all things are made from water. Plato believed that the shape of water is an icosahedron – flowing easily compared to the cube-shaped earth.
The theory of the Humorism, four bodily humors associated water with phlegm, as being cold and moist. The Water (classical element), classical element of water was also one of the Five elements (Chinese philosophy), five elements in traditional Chinese philosophy (along with earth (classical element), earth, fire (classical element), fire, wood (classical element), wood, and metal (classical element), metal).
Some traditional and popular Asian philosophy, Asian philosophical systems take water as a role-model. James Legge's 1891 translation of the ''Dao De Jing'' states, "The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao" and "There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed." ''Guanzi (text), Guanzi'' in the "Shui di" 水地 chapter further elaborates on the symbolism of water, proclaiming that "man is water" and attributing natural qualities of the people of different Chinese regions to the character of local water resources.
The Ancient Greek philosopher Empedocles saw Water (classical element), water as one of the four classical elements (along with fire, earth, and Air (classical element), air), and regarded it as an ylem, or basic substance of the universe. Thales, whom Aristotle portrayed as an astronomer and an engineer, theorized that the earth, which is denser than water, emerged from the water. Thales, a monist, believed further that all things are made from water. Plato believed that the shape of water is an icosahedron – flowing easily compared to the cube-shaped earth.
The theory of the Humorism, four bodily humors associated water with phlegm, as being cold and moist. The Water (classical element), classical element of water was also one of the Five elements (Chinese philosophy), five elements in traditional Chinese philosophy (along with earth (classical element), earth, fire (classical element), fire, wood (classical element), wood, and metal (classical element), metal).
Some traditional and popular Asian philosophy, Asian philosophical systems take water as a role-model. James Legge's 1891 translation of the ''Dao De Jing'' states, "The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao" and "There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed." ''Guanzi (text), Guanzi'' in the "Shui di" 水地 chapter further elaborates on the symbolism of water, proclaiming that "man is water" and attributing natural qualities of the people of different Chinese regions to the character of local water resources.