|
Vapor
In physics, a vapor (American English) or vapour (Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensation, condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist at any pressure. In the Earth's atmosphere, atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vapor Pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions. The vapor p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Water Vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the Sublimation (phase transition), sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds and fog. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide and methane. Use of water vapor, as steam, has been important for cooking, and as a major component in energy prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vapor Being Used In A Cloud Chamber
In physics, a vapor (American English) or vapour (Commonwealth English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist at any pressure. In the atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). When this is true, the two phases will be in equilibrium, and the gas-partial pressure will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still. Distillation can operate over a wide range of pressures from 0.14 bar (e.g., ethylbenzene/ styrene) to nearly 21 bar (e.g., propylene/propane) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 ( o-xylene/ m-xylene) to 81.2 (water/ ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). In respiratory physiology, the partial pressure of a dissolved gas in liquid (such as oxygen in arterial blood) is also defined as the partial pressure of that gas as it would be undissolved in gas phase yet in equilibrium with the liquid. This concept is also known as blood gas tension. In this sense, the diffusion of a gas liquid is said to be driven by differences in partial pressure (not concentration). In chemistry and thermodynamics, this concept is generalized to non-ideal gases and instead called fugacity. The partial pressure of a gas is a measure of its Thermodynamic activity, thermodynamic activity. Gases dissolve, diffuse, and react accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
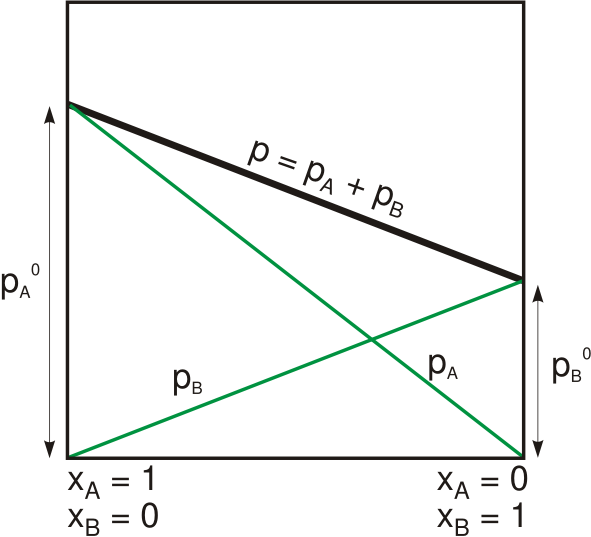
Raoult's Law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is equal to the vapor pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution. Mathematically, Raoult's law for a single component in an ideal solution is stated as : p_i = p_i^\star x_i where p_i is the partial pressure of the component i in the gaseous mixture above the solution, p_i^\star is the equilibrium vapor pressure of the pure component i, and x_i is the mole fraction of the component i in the liquid or solid solution. Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Normal Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen Dioxide Gas
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers. Nitrogen dioxide is poisonous and can be fatal if inhaled in large quantities. Cooking with a gas stove produces nitrogen dioxide which causes poorer indoor air quality. Combustion of gas can lead to increased concentrations of nitrogen dioxide throughout the home environment which is linked to respiratory issues and diseases. The LC50 (median lethal dose) for humans has been estimated to be 174 ppm for a 1-hour exposure. It is also included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above and becomes a yellowish-brown liquid below . It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Condensation is usually associated with water. Initiation Condensation is initiated by the formation of atomic/molecular clusters of that species within its gaseous volume—like rain drop or snow flake formation within clouds—or at the contact between such gaseous phase and a liquid or solid surface. In clouds, this can be catalyzed by water-nucleating proteins, produced by atmospheric microbes, which are capable of binding gaseous or liquid water molecules. Reversibility scenarios A few distinct rev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Atmosphere (unit)
The standard atmosphere (symbol: atm) is a unit of pressure defined as Pa. It is sometimes used as a ''reference pressure'' or ''standard pressure''. It is approximately equal to Earth's average atmospheric pressure at sea level. History The standard atmosphere was originally defined as the pressure exerted by a 760 mm column of mercury at and standard gravity (''g''n = ). It was used as a reference condition for physical and chemical properties, and the definition of the centigrade temperature scale set 100 °C as the boiling point of water at this pressure. In 1954, the 10th General Conference on Weights and Measures (CGPM) adopted ''standard atmosphere'' for general use and affirmed its definition of being precisely equal to dynes per square centimetre (). This defined pressure in a way that is independent of the properties of any particular substance. In addition, the CGPM noted that there had been some misapprehension that the previous definition (from the 9th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Binary Boiling Point Diagram New
Binary may refer to: Science and technology Mathematics * Binary number, a representation of numbers using only two values (0 and 1) for each digit * Binary function, a function that takes two arguments * Binary operation, a mathematical operation that takes two arguments * Binary relation, a relation involving two elements * Finger binary, a system for counting in binary numbers on the fingers of human hands Computing * Binary code, the representation of text and data using only the digits 1 and 0 * Bit, or binary digit, the basic unit of information in computers * Binary file, composed of something other than human-readable text ** Executable, a type of binary file that contains machine code for the computer to execute * Binary tree, a computer tree data structure in which each node has at most two children * Binary-coded decimal, a method for encoding for decimal digits in binary sequences Astronomy * Binary star, a star system with two stars in it * Binary planet, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Crepuscular Rays Beam Through The Mist Blown From Takkakaw Falls
In zoology, a crepuscular animal is one that is active primarily during the twilight period, being matutinal (active during dawn), vespertine/vespertinal (active during dusk), or both. This is distinguished from diurnal and nocturnal behavior, where an animal is active during the hours of daytime and of night, respectively. Some crepuscular animals may also be active by moonlight or during an overcast day. Matutinal animals are active only after dawn, and vespertine only before dusk. A number of factors affect the time of day an animal is active. Predators hunt when their prey is available, and prey try to avoid the times when their principal predators are at large. The temperature may be too high at midday or too low at night. Some creatures may adjust their activities depending on local competition. Etymology and usage The word ''crepuscular'' derives from the Latin '' crepusculum'' ("twilight"). Its sense accordingly differs from diurnal and nocturnal behavior, which re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |