Hydrogenation disproportionation desorption and recombination on:
[Wikipedia]
[Google]
[Amazon]
 Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to
File:Dichlorotris(triphenylphosphine)ruthenium(II).png,
 Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
File:CarvoneH2.png, Selective hydrogenation of the less hindered alkene group in carvone using a homogeneous catalyst ( Wilkinson's catalyst).
File:PhC2HH2.png, Partial hydrogenation of phenylacetylene using the
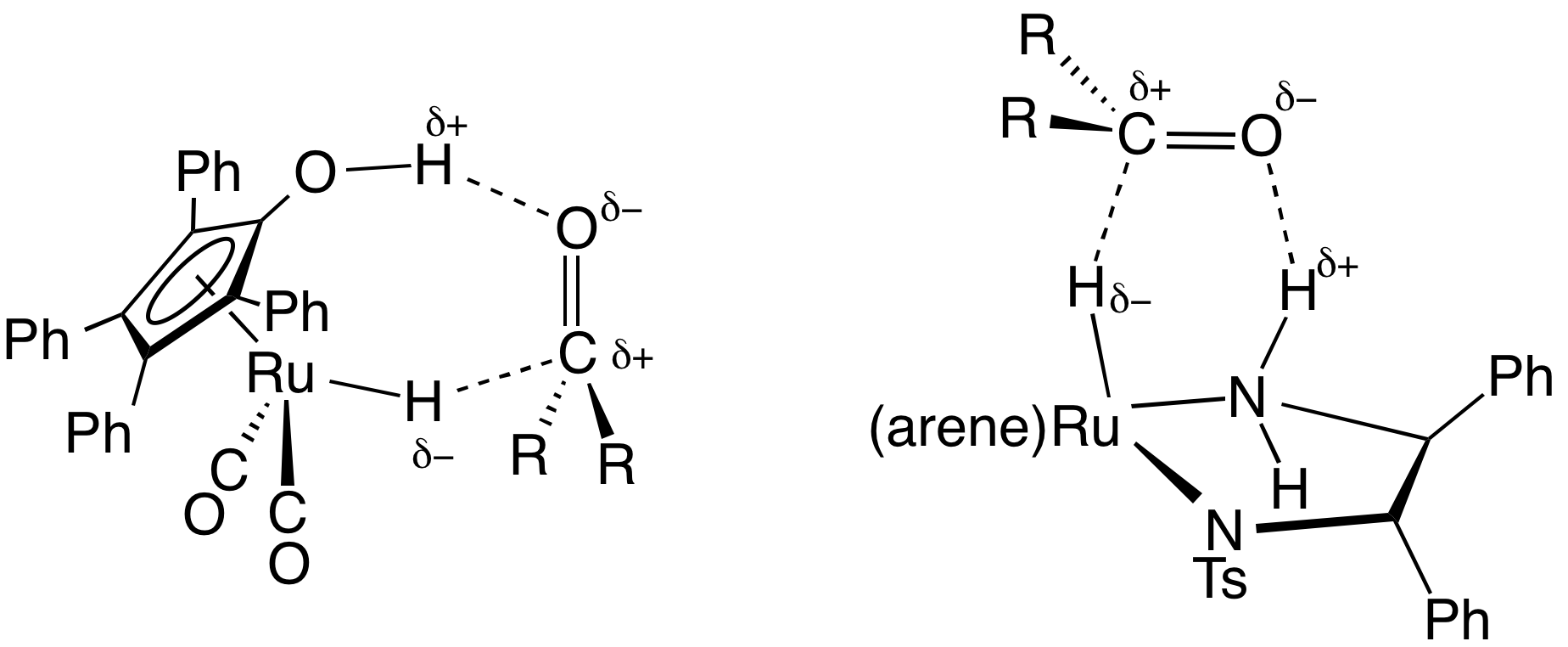 ''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as solvents for the reaction, include
''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as solvents for the reaction, include
RCH=CH2 + D2 -> RCHDCH2D
L_\mathitM + H2 -> L_\mathitMH2
* binding of alkene:
*:L_\mathitM(\eta^2 H2) + CH2=CHR -> L_MH2(CH2=CHR) + L
* transfer of one hydrogen atom from the metal to carbon (migratory insertion)
*:L_MH2(CH2=CHR) -> L_M(H)(CH2-CH2R)
* transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
*:L_M(H)(CH2-CH2R) -> L_M + CH3-CH2R
\underset + \underset -> ce350-550^\circ\ce C] \underset
Oxygen can be partially hydrogenated to give hydrogen peroxide, although this process has not been commercialized. One difficulty is preventing the catalysts from triggering decomposition of the hydrogen peroxide to form water.
 A chemical kinetics study found this reaction is
A chemical kinetics study found this reaction is  The reduction of
The reduction of
high pressure hydrogen generators
which generate hydrogen up to 100 bar (1400 PSI) from water. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
Organic Syntheses, Coll. Vol. 7, p.226 (1990).
*
Organic Syntheses, Coll. Vol. 8, p.609 (1993).
*
Organic Syntheses, Coll. Vol. 5, p.552 (1973).
*
Organic Syntheses, Coll. Vol. 3, p.720 (1955).
*
Organic Syntheses, Coll. Vol. 6, p.371 (1988).
* early work on transfer hydrogenation: ** ** **
"The Magic of Hydro"
'' Popular Mechanics'', June 1931, pp. 107–109 – early article for the general public on hydrogenation of oil produced in the 1930s {{Authority control Addition reactions Homogeneous catalysis Industrial processes Hydrogen Organic redox reactions Oil refining Oil shale technology Synthetic fuel technologies
reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox react ...
or saturate
Saturate may refer to:
* ''Saturate'' (Breaking Benjamin album), 2002
* ''Saturate'' (Gojira album), 1999
* ''Saturate'' (Jeff Deyo album), 2002
* " Electronic Battle Weapon 8", a song by The Chemical Brothers, a shorter version of which was re ...
organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* Th ...
and triple
Triple is used in several contexts to mean "threefold" or a "treble":
Sports
* Triple (baseball), a three-base hit
* A basketball three-point field goal
* A figure skating jump with three rotations
* In bowling terms, three strikes in a row
* In ...
bonds in hydrocarbons.
Process
Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.Related or competing reactions
The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis to trans. This process is of great interest because hydrogenation technology generates most of the trans fat in foods (see below). A reaction where bonds are broken while hydrogen is added is calledhydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
, a reaction that may occur to carbon-carbon and carbon-heteroatom ( oxygen, nitrogen or halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) bonds. Some hydrogenations of polar bonds are accompanied by hydrogenolysis.
Hydrogen sources
For hydrogenation, the obvious source of hydrogen is H2 gas itself, which is typically available commercially within the storage medium of a pressurized cylinder. The hydrogenation process often uses greater than 1 atmosphere of H2, usually conveyed from the cylinders and sometimes augmented by "booster pumps". Gaseous hydrogen is produced industrially from hydrocarbons by the process known as steam reforming.Paul N. Rylander, "Hydrogenation and Dehydrogenation" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2005. For many applications, hydrogen is transferred from donor molecules such as formic acid, isopropanol, anddihydroanthracene
9,10-Dihydroanthracene is an organic compound that is derived from the polycyclic aromatic hydrocarbon anthracene. Several isomers of dihydroanthracene are known, but the 9,10 derivative is most common. It is a colourless solid that is used as a ...
. These hydrogen donors undergo dehydrogenation to, respectively, carbon dioxide, acetone, and anthracene. These processes are called transfer hydrogenations.
Substrates
An important characteristic of alkene and alkyne hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with "syn addition
In organic chemistry, syn- and anti-addition are different ways in which substituent molecules can be added to an alkene or alkyne. The concepts of syn and anti addition are used to characterize the different reactions of organic chemistry by ref ...
", with hydrogen entering from the least hindered side. This reaction can be performed on a variety of different functional groups.
Catalysts
With rare exceptions, H2 is unreactive toward organic compounds in the absence of metal catalysts. The unsaturated substrate is chemisorbed onto the catalyst, with most sites covered by the substrate. In heterogeneous catalysts, hydrogen forms surface hydrides (M-H) from which hydrogens can be transferred to the chemisorbed substrate. Platinum, palladium, rhodium, and ruthenium form highly active catalysts, which operate at lower temperatures and lower pressures of H2. Non-precious metal catalysts, especially those based on nickel (such as Raney nickel andUrushibara nickel Urushibara nickel is a nickel based hydrogenation catalyst, named after Yoshiyuki Urushibara.
History
It was discovered by Yoshiyuki Urushibara in 1951, while doing research on the reduction of estrone to estradiol.
Preparation
First nickel is pr ...
) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:
Catalysts are usually classified into two broad classes: homogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
s and heterogeneous catalysts. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
Homogeneous catalysts
Some well known homogeneous catalysts are indicated below. These are coordination complexes that activate both the unsaturated substrate and the H2. Most typically, these complexes contain platinum group metals, especially Rh and Ir.Dichlorotris(triphenylphosphine)ruthenium(II)
Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used i ...
is a precatalyst based on ruthenium.
File:Crabtree.svg, Crabtree's catalyst is a highly active catalyst featuring iridium.
File:Cyclooctadiene-rhodium-chloride-dimer-2D-skeletal.png, Rh2Cl2(cod)2 is a precursor to many homogeneous catalysts.
File:(S)-iPr-PHOX.svg, (S)-iPr-PHOX
(''S'')-iPr-PHOX, or (''S'')-2- -(diphenylphosphino)phenyl4-isopropyl-4,5-dihydrooxazole, is a chiral, bidentate, ligand derived from the amino alcohol valinol. It is part of a broader class of phosphinooxazolines ligands and has found applicat ...
is a typical chelating phosphine ligand used in asymmetric hydrogenation.
 Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Homogeneous catalysts are also used in asymmetric synthesis by the hydrogenation of prochiral substrates. An early demonstration of this approach was the Rh-catalyzed hydrogenation of enamides as precursors to the drug L-DOPA. To achieve asymmetric reduction, these catalyst are made chiral by use of chiral diphosphine ligands. Rhodium catalyzed hydrogenation has also been used in the herbicide production of S-metolachlor, which uses a Josiphos type ligand (called Xyliphos). In principle asymmetric hydrogenation can be catalyzed by chiral heterogeneous catalysts, but this approach remains more of a curiosity than a useful technology.
Heterogeneous catalysts
Heterogeneous catalysts for hydrogenation are more common industrially. In industry, precious metal hydrogenation catalysts are deposited from solution as a fine powder on the support, which is a cheap, bulky, porous, usually granular material, such asactivated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
, alumina, calcium carbonate or barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium an ...
. For example, platinum on carbon is produced by reduction of chloroplatinic acid
Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula 3Osub>2 tCl6H2O)''x'' (0 ≤ ''x'' ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Alth ...
''in situ'' in carbon. Examples of these catalysts are 5% ruthenium on activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
, or 1% platinum on alumina. Base metal catalysts, such as Raney nickel, are typically much cheaper and do not need a support. Also, in the laboratory, unsupported (massive) precious metal catalysts such as platinum black are still used, despite the cost.
As in homogeneous catalysts, the activity is adjusted through changes in the environment around the metal, i.e. the coordination sphere. Different faces of a crystalline heterogeneous catalyst display distinct activities, for example. This can be modified by mixing metals or using different preparation techniques. Similarly, heterogeneous catalysts are affected by their supports.
In many cases, highly empirical modifications involve selective "poisons". Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes to alkenes using Lindlar's catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. w ...
. For example, when the catalyst palladium is placed on barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium an ...
and then treated with quinoline, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of phenylacetylene to styrene.
Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. ...
.
File:ImineH2.png, Hydrogenation of an imine using a Raney nickel catalyst, a popular heterogeneous catalyst.
File:ResorcinolH2.png, Partial hydrogenation of a resorcinol derivative using a Raney-Nickel catalyst.
File:SuccPdH2.png, Hydrogenation of maleic acid to succinic acid.
Transfer hydrogenation
 ''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as solvents for the reaction, include
''Transfer hydrogenation'' uses hydrogen-donor molecules other than molecular H2. These "sacrificial" hydrogen donors, which can also serve as solvents for the reaction, include hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
, and alcohols such as isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
.
In organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, transfer hydrogenation is useful for the asymmetric hydrogenation of polar unsaturated substrates, such as ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, aldehydes and imines, by employing chiral catalysts
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
.
Electrolytic hydrogenation
Polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
substrates such as nitriles can be hydrogenated electrochemically
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, using protic solvents and reducing equivalents as the source of hydrogen.
Thermodynamics and mechanism
The addition of hydrogen to double or triple bonds in hydrocarbons is a type of redox reaction that can be thermodynamically favorable. For example, the addition of hydrogen to ethene has a Gibbs free energy change of -101 kJ·mol−1, which is highlyexothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
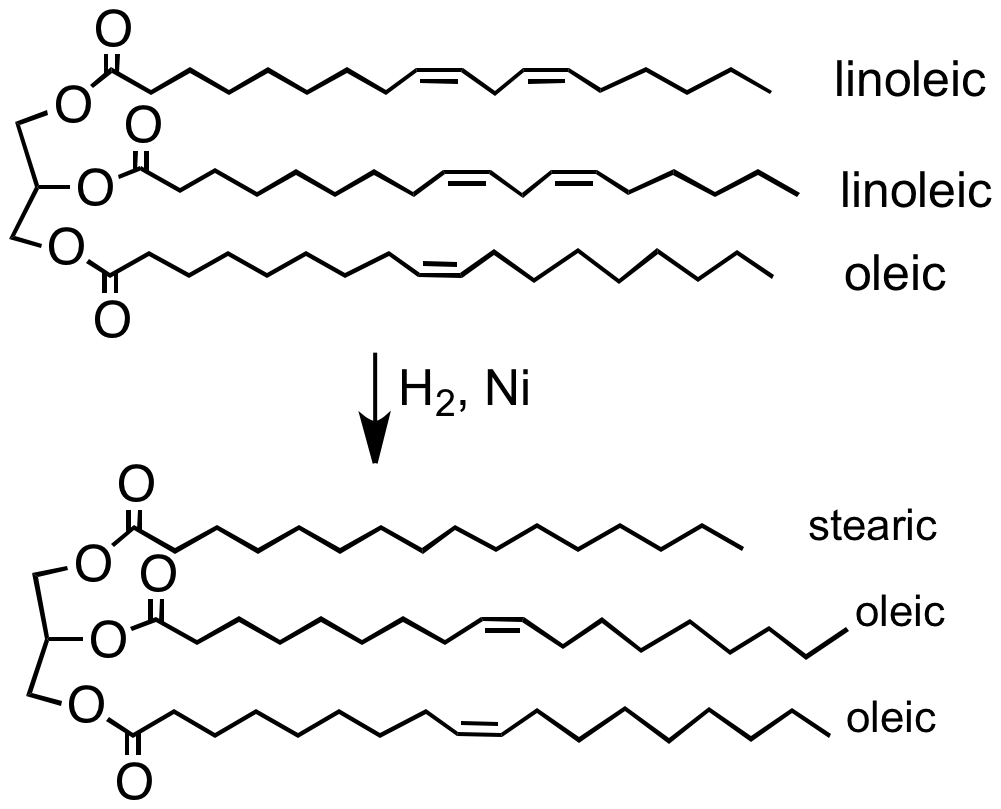
. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kJ/mol), is sufficient to raise the temperature of the oil by 1.6–1.7 °C per iodine number drop.
However, the reaction rate for most hydrogenation reactions is negligible in the absence of catalysts. The mechanism
Mechanism may refer to:
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that a ...
of metal-catalyzed hydrogenation of alkenes and alkynes has been extensively studied. First of all isotope labeling using deuterium confirms the regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
of the addition:
:Heterogeneous catalysis
On solids, the accepted mechanism is the Horiuti- Polanyi mechanism: # Binding of the unsaturated bond # Dissociation of on the catalyst # Addition of one atom of hydrogen; this step is reversible # Addition of the second atom; effectively irreversible. In the third step, the alkyl group can revert to alkene, which can detach from the catalyst. Consequently, contact with a hydrogenation catalyst allows ''cis-trans''-isomerization. The ''trans''-alkene can reassociate to the surface and undergo hydrogenation. These details are revealed in part using D2 (deuterium), because recovered alkenes often contain deuterium. For aromatic substrates, the first hydrogenation is slowest. The product of this step is a cyclohexadiene, which hydrogenate rapidly and are rarely detected. Similarly, the cyclohexene is ordinarily reduced to cyclohexane.Homogeneous catalysis
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal(H)2 complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps: * binding of the hydrogen to give a dihydride complex via oxidative addition (preceding the oxidative addition of is the formation of a dihydrogen complex): *:Inorganic substrates
The hydrogenation of nitrogen to give ammonia is conducted on a vast scale by theHaber–Bosch
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
process, consuming an estimated 1% of the world's energy supply.
:Industrial applications
Catalytic hydrogenation has diverse industrial uses. Most frequently, industrial hydrogenation relies on heterogeneous catalysts.Food industry
The food industry hydrogenates vegetable oils to convert them into solid or semi-solid fats that can be used in spreads, candies, baked goods, and other products likemargarine
Margarine (, also , ) is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was orig ...
. Vegetable oils are made from polyunsaturated fatty acids (having more than one carbon-carbon double bond). Hydrogenation eliminates some of these double bonds.Ian P. Freeman "Margarines and Shortenings" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim.
:
Petrochemical industry
In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. Relevant to liquid fuels that are stored sometimes for long periods in air, saturated hydrocarbons exhibit superior storage properties. On the other hand, alkenes tend to formhydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have t ...
s, which can form gums that interfere with fuel handling equipment. For example, mineral turpentine
White spirit (AU, UK & Ireland)Primarily in the United Kingdom and Australia. In New Zealand "white spirit" can also refer to Coleman fuel (white gas). or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ), turpentine substit ...
is usually hydrogenated. Hydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of ...
of heavy residues into diesel is another application. In isomerization and catalytic reforming processes, some hydrogen pressure is maintained to hydrogenolyze coke formed on the catalyst and prevent its accumulation.
Organic chemistry
Hydrogenation is a useful means for converting unsaturated compounds into saturated derivatives. Substrates include not only alkenes and alkynes, but also aldehydes, imines, and nitriles, which are converted into the corresponding saturated compounds, i.e. alcohols and amines. Thus, alkyl aldehydes, which can be synthesized with the oxo process from carbon monoxide and an alkene, can be converted to alcohols. E.g.1-propanol
Propan-1-ol (also propanol, n-propyl alcohol) is a primary alcohol with the formula and sometimes represented as PrOH or ''n''-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many ferme ...
is produced from propionaldehyde, produced from ethene and carbon monoxide. Xylitol, a polyol, is produced by hydrogenation of the sugar xylose
Xylose ( grc, ξύλον, , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional gro ...
, an aldehyde. Primary amines can be synthesized by hydrogenation of nitriles, while nitriles are readily synthesized from cyanide and a suitable electrophile. For example, isophorone diamine, a precursor to the polyurethane monomer isophorone diisocyanate
Isophorone diisocyanate (IPDI) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is produced in relatively small quantities, accounting for (with hexamethylene diisocyanate) only 3.4% ...
, is produced from isophorone nitrile by a tandem nitrile hydrogenation/reductive amination by ammonia, wherein hydrogenation converts both the nitrile into an amine and the imine formed from the aldehyde and ammonia into another amine.
Hydrogenation of coal
History
Heterogeneous catalytic hydrogenation
The earliest hydrogenation is that of platinum catalyzed addition of hydrogen to oxygen in the Döbereiner's lamp, a device commercialized as early as 1823. The French chemist Paul Sabatier is considered the father of the hydrogenation process. In 1897, building on the earlier work of James Boyce, an American chemist working in the manufacture of soap products, he discovered that traces of nickel catalyzed the addition of hydrogen to molecules of gaseous hydrocarbons in what is now known as theSabatier process
The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures (optimally 300–400 °C) and pressures (perhaps 3 MPa ) in the presence of a nickel catalyst. It w ...
. For this work, Sabatier shared the 1912 Nobel Prize in Chemistry. Wilhelm Normann was awarded a patent in Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a worldwide industry. The commercially important Haber–Bosch process, first described in 1905, involves hydrogenation of nitrogen. In the Fischer–Tropsch process, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
In 1922, Voorhees and Adams described an apparatus for performing hydrogenation under pressures above one atmosphere. The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on Voorhees and Adams' research and remains in widespread use. In 1924 Murray Raney
Murray Raney (October 14, 1885 – March 3, 1966) was an American mechanical engineer born in Carrollton, Kentucky. He was the developer of a nickel catalyst that became known as Raney nickel, which is often used in industrial processes and scie ...
developed a finely powdered form of nickel, which is widely used to catalyze hydrogenation reactions such as conversion of nitriles to amines or the production of margarine.
Homogeneous catalytic hydrogenation
In the 1930s, Calvin discovered that copper(II) complexes oxidized H2. The 1960s witnessed the development of well definedhomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
s using transition metal complexes, e.g., Wilkinson's catalyst (RhCl(PPh3)3). Soon thereafter cationic Rh and Ir were found to catalyze the hydrogenation of alkenes and carbonyls. In the 1970s, asymmetric hydrogenation was demonstrated in the synthesis of L-DOPA, and the 1990s saw the invention of Noyori asymmetric hydrogenation. The development of homogeneous hydrogenation was influenced by work started in the 1930s and 1940s on the oxo process and Ziegler–Natta polymerization.
Metal-free hydrogenation
For most practical purposes, hydrogenation requires a metal catalyst. Hydrogenation can, however, proceed from some hydrogen donors without catalysts, illustrative hydrogen donors being diimide andaluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-''i''-Pr)3, where ''i''-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis.
Structure
A tetrameric st ...
, the latter illustrated by the Meerwein–Ponndorf–Verley reduction. Some metal-free catalytic systems have been investigated in academic research. One such system for reduction of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s consists of ''tert''-butanol and potassium tert-butoxide and very high temperatures. The reaction depicted below describes the hydrogenation of benzophenone:
:first-order
In mathematics and other formal sciences, first-order or first order most often means either:
* "linear" (a polynomial of degree at most one), as in first-order approximation and other calculus uses, where it is contrasted with "polynomials of high ...
in all three reactants suggesting a cyclic 6-membered transition state.
Another system for metal-free hydrogenation is based on the phosphine- borane, compound 1, which has been called a '' frustrated Lewis pair''. It reversibly accepts dihydrogen at relatively low temperatures to form the phosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium ...
borate 2 which can reduce simple hindered imines.
:nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
to aniline has been reported to be catalysed by fullerene, its mono-anion, atmospheric hydrogen and UV light.
Equipment used for hydrogenation
Today's bench chemist has three main choices of hydrogenation equipment: * Batch hydrogenation under atmospheric conditions * Batch hydrogenation at elevated temperature and/or pressure * Flow hydrogenationBatch hydrogenation under atmospheric conditions
The original and still a commonly practised form of hydrogenation in teaching laboratories, this process is usually effected by adding solid catalyst to a round bottom flask of dissolved reactant which has been evacuated using nitrogen or argon gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then supplied from a H2-filled balloon. The resulting three phase mixture is agitated to promote mixing. Hydrogen uptake can be monitored, which can be useful for monitoring progress of a hydrogenation. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate or with gauges for each reaction vessel.Batch hydrogenation at elevated temperature and/or pressure
Since many hydrogenation reactions such ashydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
of protecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
and the reduction of aromatic systems proceed extremely sluggishly at atmospheric temperature and pressure, pressurised systems are popular. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source, and the pressurized slurry is mechanically rocked to provide agitation, or a spinning basket is used. Recent advances in electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
technology have led to the development ohigh pressure hydrogen generators
which generate hydrogen up to 100 bar (1400 PSI) from water. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
Flow hydrogenation
Flow hydrogenation has become a popular technique at the bench and increasingly the process scale. This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen. Using establishedHPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
technology, this technique allows the application of pressures from atmospheric to . Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering pyrophoric catalysts.
Industrial reactors
Catalytic hydrogenation is done in a tubular plug-flow reactor (PFR) packed with a supported catalyst. The pressures and temperatures are typically high, although this depends on the catalyst. Catalyst loading is typically much lower than in laboratory batch hydrogenation, and various promoters are added to the metal, or mixed metals are used, to improve activity, selectivity and catalyst stability. The use of nickel is common despite its low activity, due to its low cost compared to precious metals. Gas Liquid Induction Reactors (Hydrogenator) are also used for carrying out catalytic hydrogenation.See also
* Carbon neutral fuel * Dehydrogenation *H-Bio H-Bio is an oil-refining processes which involves converting vegetable oil into high-quality diesel via hydrogenation. Hydrogenation is a chemical reaction, in which a substance is treated with Hydrogen, thus resulting in a new product. In H-Bio, H ...
* Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
* Hydrodesulfurization
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of remov ...
, hydrotreater and oil desulfurization
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to desulfurization, remove sulfur (S) from natural gas and from oil refinery, refined petroleum products, such as gasoline, gasoline or petrol, jet fuel, kerosene, diesel fue ...
* Josiphos ligands
* Timeline of hydrogen technologies
* Transfer hydrogenation
* Rhodium-catalyzed hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
* Trans fats
Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial p ...
References
Further reading
* * examples of hydrogenation from Organic Syntheses: *Organic Syntheses, Coll. Vol. 7, p.226 (1990).
*
Organic Syntheses, Coll. Vol. 8, p.609 (1993).
*
Organic Syntheses, Coll. Vol. 5, p.552 (1973).
*
Organic Syntheses, Coll. Vol. 3, p.720 (1955).
*
Organic Syntheses, Coll. Vol. 6, p.371 (1988).
* early work on transfer hydrogenation: ** ** **
External links
"The Magic of Hydro"
'' Popular Mechanics'', June 1931, pp. 107–109 – early article for the general public on hydrogenation of oil produced in the 1930s {{Authority control Addition reactions Homogeneous catalysis Industrial processes Hydrogen Organic redox reactions Oil refining Oil shale technology Synthetic fuel technologies